News & Views |
Featured
-
-
Letter |
 Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia
Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia
Multi-isotope imaging mass spectrometry is used to quantify protein turnover in animal stereocilia, showing that rapid turnover occurs only in stereocilia tips.
- Duan-Sun Zhang
- , Valeria Piazza
- & Claude P. Lechene
-
Research Highlights |
No centrosome, no problem
-
Research Highlights |
The first microtubules
-
Letter |
 Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A
Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A
- A. J. Ehrlicher
- , F. Nakamura
- & T. P. Stossel
-
Research Highlights |
Microtubules beat in sync
-
Article |
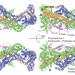 Structure and control of the actin regulatory WAVE complex
Structure and control of the actin regulatory WAVE complex
In cells, WAVE protein, a central regulator of actin dynamics during cell motility, is constitutively incorporated into WAVE regulatory complex (WRC), is normally present in an inactive state and can be activated by a number of inputs. These authors present the structure and mechanistic analysis of WRC. The combined data reveal how the WAVE protein is inhibited within the WRC complex and provide mechanisms for WRC activation at the plasma membrane.
- Zhucheng Chen
- , Dominika Borek
- & Michael K. Rosen
-
Letter |
 Tension directly stabilizes reconstituted kinetochore-microtubule attachments
Tension directly stabilizes reconstituted kinetochore-microtubule attachments
The kinetochore is a large protein complex that assembles on centromeric DNA and captures microtubules to mediate chromosome separation. These authors report the first purification of functional kinetochores. They also show that kinetochore particles maintain load-bearing associations with assembling and disassembling ends of single microtubules and that tension increases the lifetimes of the attachments directly. These results provide evidence that tension selectively stabilises kinetochore–microtubule interactions.
- Bungo Akiyoshi
- , Krishna K. Sarangapani
- & Sue Biggins
-
Letter |
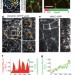 Planar polarized actomyosin contractile flows control epithelial junction remodelling
Planar polarized actomyosin contractile flows control epithelial junction remodelling
Here, germ-band extension in Drosophila is studied in which epithelial cells undergo an ordered process of intercalation resulting in tissue extension through remodelling of cell junctions. Cell junction shrinkage is driven by polarized flow of medial Myosin-II pulses towards junctions which organizes the whole process of intercalation. The flow of Myosin II is driven by the polarized distribution of E-cadherin complexes at adherens junctions. Thus, epithelial morphogenesis is driven by polarized contractile actomyosin flows emerging from interactions between E-cadherin and actomyosin networks.
- Matteo Rauzi
- , Pierre-François Lenne
- & Thomas Lecuit
-
-
Letter |
 Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1
Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1
Entry of herpes simplex virus-1 into cells requires cellular receptors for both envelope glycoprotein B and envelope glycoprotein D. These authors show that the interaction of non-muscle myosin heavy chain IIA with envelope glycoprotein B is important for entry of herpes simplex virus-1.
- Jun Arii
- , Hideo Goto
- & Yasushi Kawaguchi
-
Article |
 Video imaging of walking myosin V by high-speed atomic force microscopy
Video imaging of walking myosin V by high-speed atomic force microscopy
High-speed atomic force microscopy can be used to record the structure and dynamics of biomolecules simultaneously. These authors use this method to directly observe the dynamics of the motor protein myosin V moving along actin filaments, with unprecedented time resolution. The high-resolution movies provide evidence supporting the 'swinging lever-arm' model of myosin motility, and provide important insights into the mechanism of motor movement.
- Noriyuki Kodera
- , Daisuke Yamamoto
- & Toshio Ando
-
Research Highlights |
Molecular biology: Proteins actin' differently
-
News |
 Nanotubes help cells pass messages
Nanotubes help cells pass messages
Actin cables transmit electrical signals between cells.
- Amy Maxmen
-
Letter |
 Asterless is a scaffold for the onset of centriole assembly
Asterless is a scaffold for the onset of centriole assembly
Centrioles are essential for the formation of centrosomes, cilia and flagella. The centriolar protein Polo-like-kinase 4 (Plk4) is a key regulator of centriole biogenesis and for maintaining constant centriole number in cells. These authors show that the centriolar protein Asterless (CEP152 in humans) interacts with Plk4 and Sas-4. They find that Asl functions as a scaffold for Plk4 and Sas-4 that facilitates self-assembly and duplication of the centriole, and organization of pericentriolar material.
- Nikola S. Dzhindzhev
- , Quan D. Yu
- & David M. Glover
-
Letter |
 Direct visualization of secondary structures of F-actin by electron cryomicroscopy
Direct visualization of secondary structures of F-actin by electron cryomicroscopy
The formation of filamentous F-actin, through polymerization of globular G-actin, is essential for processes such as cell motility and muscle contraction. These authors report the structure of F-actin as visualized by electron cryomicroscopy, and build a complete atomic model of F-actin. This new structure will improve our understanding of the mechanism of actin assembly and disassembly.
- Takashi Fujii
- , Atsuko H. Iwane
- & Keiichi Namba
-
Letter |
 MEC-17 is an α-tubulin acetyltransferase
MEC-17 is an α-tubulin acetyltransferase
In eukaryotic cells, a subset of microtubules undergo post-translational modifications such as acetylation, which alters microtubule dynamics and trafficking of motors. These authors identify MEC-17 as the enzyme that directly acetylates α-tubulin in vitro and in vivo and in both invertebrates and vertebrates. This is the identification of the long-sought enzyme that acetylates microtubules.
- Jyothi S. Akella
- , Dorota Wloga
- & Jacek Gaertig
-
Letter |
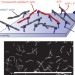 Polar patterns of driven filaments
Polar patterns of driven filaments
Collective motion is a ubiquitous self-organization phenomenon that can be observed in systems ranging from flocks of animals to the cytoskeleton. Similarities between these systems suggest that there are universal underlying principles. This idea can be tested with 'active' or 'driven' fluids, but so far such systems have offered limited parameter control. Here, an active fluid is studied that contains only a few components — actin filaments and molecular motors — allowing the control of all relevant system parameters.
- Volker Schaller
- , Christoph Weber
- & Andreas R. Bausch
-
Letter |
 Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
XXXMicrotubules are nucleated in vivo by γ-tubulin complexes and comprise 13 protofilaments. How this precise geometry is controlled remains unclear. These authors report the cryo-electron microscopic structure of the universally conserved, core microtubule nucleating complex, γ-tubulin small complex. The structure provides insight into how this complex establishes thirteen-fold tubulin symmetry.
- Justin M. Kollman
- , Jessica K. Polka
- & David A. Agard
-
Letter |
 Myosin II contributes to cell-scale actin network treadmilling through network disassembly
Myosin II contributes to cell-scale actin network treadmilling through network disassembly
Eukaryotic cells crawl through a process in which the front of the cell is propelled forwards by the force provided by polymerization of actin filaments. These must be disassembled at the rear of the cell to allow sustained motility. It is now shown that non-muscle myosin II protein is needed for the disassembly of actin networks at the rear of crawling cells.
- Cyrus A. Wilson
- , Mark A. Tsuchida
- & Julie A. Theriot
-
Letter |
 Functional genomic screen for modulators of ciliogenesis and cilium length
Functional genomic screen for modulators of ciliogenesis and cilium length
Primary cilia are tiny hair-like structures expressed on the surface of eukaryotic cells. They participate in a range of processes, such as sensing the extracellular environment and regulating signalling pathways during development. Here, a functional genomic screen is presented that used RNA interference to identify human genes involved in controlling ciliogenesis. Several positive and negative ciliogenesis modulators with broad-ranging functions were found.
- Joon Kim
- , Ji Eun Lee
- & Joseph G. Gleeson
-
News & Views |
 Actin filaments up against a wall
Actin filaments up against a wall
The front of motile cells is thought to be pushed out by branched filaments of actin protein abutting the cell membrane. New work challenges this textbook view, showing that actin branches grow away from, or obliquely to, a surface.
- Cécile Sykes
- & Julie Plastino
-
Letter |
 Mical links semaphorins to F-actin disassembly
Mical links semaphorins to F-actin disassembly
Semaphorins and their receptors, plexins, relay guidance information to neurons during development and regulate actin dynamics through an unknown mechanism. Recently, proteins of the Mical family of enzymes have been found to associate with plexins; here, Mical is reported to directly link semaphorins and their plexin receptors to the precise control of actin filament dynamics.
- Ruei-Jiun Hung
- , Umar Yazdani
- & Jonathan R. Terman
-
-
News & Views |
 How cilia beat
How cilia beat
Physics provides new approaches to difficult biological problems: a plausible mathematical model of how cilia and flagella beat has been formulated, but it needs to be subjected to rigorous experimental tests.
- T. J. Mitchison
- & H. M. Mitchison

 A staggering giant
A staggering giant Myosin in motion
Myosin in motion Cell mechanics and the cytoskeleton
Cell mechanics and the cytoskeleton