Abstract
The wide tunability of strongly correlated transition metal (TM) oxides stems from their complex electronic properties and the coupled degrees of freedom. Among the perovskite oxides family, LaMO3 (M = Ti-Ni) allows an M-dependent systematic study of the electronic structure within the same-structure-family motif. While most of the studies have been focusing on the 3d TMs and oxygen sites, the role of the rare-earth site has been far less explored. In this work, we use resonant inelastic X-ray scattering (RIXS) at the lanthanum N4,5 edges and density functional theory (DFT) to investigate the hybridization mechanisms in LaMO3. We link the spatial-overlap-driven hybridization to energetic-overlap-driven hybridization by comparing the RIXS chemical shifts and the DFT band widths. The scope is extended to highly covalent Ruddlesden-Popper perovskite La2CuO4 by intercalating lanthanum atoms to rock-salt layers. Our work evidences an observable contribution of localized lanthanum 5p and 4f orbitals in the band structure.
Similar content being viewed by others
Introduction
Transition metal (TM) perovskite oxides [ABO3, A = rare earth (RE) cation, B = TM cation] are canonical strongly correlated ternary oxides. They have drawn considerable scientific interest due to their rich phase diagrams resulting from coupled orbital, spin, lattice, and charge degrees of freedom1,2,3,4,5,6,7,8,9,10. Consequently, the transport, magnetic, and optical properties can be tuned by, e.g., doping, strain, temperature or external fields, which bring about their functionalities. Their wide applications include catalysis11,12,13, energy storage14, and electronic devices15,16 to name a few.
One of the factors that underlies the rich tunable physical properties is the orbital overlap17,18. Most of the studies of ABO3 have focused on the oxygen and TM sites, which are the dominant components of the valence band maxima and the conduction band minima19,20,21,22,23. For example, eg orbital mixing is the key to understanding the spin-, and orbital-order transition in manganites24. The 4f states of trivalent RE are generally considered to be highly localized due to the relativistic contraction25,26,27,28. Indeed, RE elements are often treated merely as geometric structure modulators or charge reservoirs in the context of complex oxides1,29. However, it has recently been suggested the RE 5d or 4f orbitals can be electronically hybridized with TM 3d30,31,32. The role of the RE has started to gain attention from the infinite layer nickelates where the hybridization between RE 5d and Ni 3d could have an influence on the Fermi surface and magnetic exchange27,33,34,35,36. In fact, it is still under debate how RE is participating in the hybridization scheme37 and whether nickelates possess similar superconducting mechanisms in comparison to the cuprates38,39.
The lanthanum perovskite oxides LaMO3 (M = Ti-Ni) are paradigmatic strongly correlated transition-metal oxides in which the physical properties are dominated by the partially filled d-states: from antiferromagnetic (AFM) Mott insulator to AFM charge-transfer insulator (TM = Ti-Fe), then to paramagnetic narrow gap insulator LaCoO3, and finally to correlated metal LaNiO340,41. Several theoretical approaches were dedicated to bridging the relationship between electronic, geometric, and magnetic structures42,43,44,45. The key frontier orbitals that determine the functionalities are mainly TM 3d, O 2p and their degree of hybridization46,47,48. Locally, the TM3+ is coordinated by 6 O2− in an octahedron and La3+ is coordinated by 12 O2− and intercalated in the cubic unit cell [Fig. 1(a)]. The TM 3d orbitals hybridize with the O 2p47 and the hybridization can be found as a general feature of 3d TM oxides48. Higher TM 3d - O 2p hybridization results in a delocalization of 3d electrons, an enhanced covalency, a lower on-site Coulomb interaction, a higher hopping term, and a broader band width. Covalency, which is associated with the hopping term in Hubbard model has a direct impact on the superexchange, which is a crucial parameter to determine the alignment of spins. For example, an ionic framework fails to describe magnetism in cuprates where covalency is significant between Cu and O49. However, the degree of involvement of La orbitals in the hybridization scheme remains ambiguous.
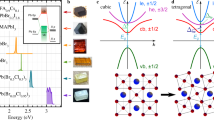
a General lanthanum perovskite oxide structure and scheme of orbital overlap based on radial wave functions. TM represents transition metal ion. b Scheme of the X-ray absorption and RIXS process in La. 3D1 and 3P1 depict the atomic terms of 4d94f1 electronic configuration. Charge transfer between ligands and metal centers is denoted as CT. c High-resolution RIXS spectra and fitted result. The purple- and green-dashed curves represent the 3D1 and 3D2 RIXS final states (electronic configuration 5p54f1), respectively. The error bars are defined by the square root of the cumulative photon counts.
Resonant inelastic X-ray scattering (RIXS) is a spectroscopic technique capable of probing the electronic structure and the elementary excitations of a material50. The coherent second-order optical process is element- and orbital-selective and has delivered important insights on a wide range of functional materials, including transition metal oxides, quantum materials, and correlated metals51,52,53,54,55. Final states with constant energy loss represent an intrinsic excitation energy. A schematic excitation of charge and orbital is illustrated in Fig. 1b.
In this work, we revisit the TM perovskite oxides hybridization scheme from the perspective of the rare-earth site. We present high-resolution RIXS experiments at the La N4,5-edges and determine the energy positions of the 3D1 state (5p54f1 configuration) in LaMO3 (M = Ti-Ni) and La2CuO4. We observe chemical shifts and we link them to the spatial and energetic overlap between TM 3d and La 4f, O 2s and La 5p. By combining the spectroscopic observables with first-principle density functional theory (DFT) calculations, we evidence the electronic hybridization between La and TM in perovskite oxides. The coupling mechanisms between La, TM, and O atoms are explained with intra- and interatomic orbital hybridizations.
Results
Figure 1a depicts an illustrative perovskite crystal structure and relative positions of the atoms in a LaMO3. The excitation and radiative decay channels are shown in Fig. 1b. Two X-ray absorption (XAS) features similar to those reported in Ref. 56 are observed, which correspond to the 3D1 and 3P1 intermediate states. Due to being well self-screened, these two multiplet features show no observable chemical sensitivity. If we resonantly excite the electrons to the intermediate 3D1 (4d94f1) state, three main radiative decay pathways are possible: (a) Elastic channel: The excited electrons relax back to the ground state. (b) Charge transfer (CT) channel: Electrons from O 2p shell refill the transient La 4d core hole. (c) Inner valence channel: A 5p electron decays via electric dipole transition and refills a 4d hole. The latter appears as a multiplet structure in the spectrum, which corresponds to atomic multiplets of the 5p54f1 configuration56,57,58. The present study exploits the chemical shifts of the energetically lowest multiplet due to the change of the chemical environment along the 3d TM perovskite oxide series.
Indeed, it has been recently evidenced that these multiplets show chemical sensitivity, which indicates that hybridizations involving La 5p and 4f atomic orbitals affect the final state energy56. In a free-ion picture, the 3D1 state is the only pure state while the rest of the multiple states are mixed of terms to some degree59. Previously, the 3D1 energy positions in a wide range of La-containing systems were determined by the LaAlO3 empirical model56. As described in Ref. 56, this model consists of a double-Voigt fit where the energy distances, the relative intensity ratios, and the intrinsic lifetime broadening are constrained and chemical broadenings are coupled. We apply this model to the perovskite compounds investigated in this work [Fig. 1c]. The model captures the lineshapes and determines the energy positions of the 3D1 RIXS final state. Moderate differences in the peak widths (0.175 – 0.268 eV, details are listed in supplementary Table S1) are attributed to different degrees of chemical broadening. We note that strain and temperature have a marginal influence on the La RIXS shift (See supplementary Fig. S1).
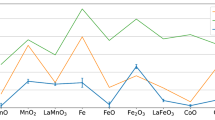
Figure 2a shows the energy of the 3D1 peak (in red) with respect to the nominal 3d electron counts on the TM site. By comparing the structurally similar perovskites, we first observe an increase of the peak energy when increasing n from n = 1 (Ti3+) to n = 6 (Co3+), i.e. when the 3d shell is half filled. From n = 6 to n = 9, as the 3d orbitals become doubly occupied and a pronounced decrease is observed. The evolution of the 3D1 peak energy vs. the number of 3d electrons is put in perspective with the TM ionic radii in Fig. 2a in blue. The effective ionic radii are based on an octahedral crystal field with a coordination number of six60. As the ionic radii are strongly dependent on the local spin configuration, both high and low spin configuration radii are shown. On one hand, as the atomic number increases, the increasing attractive Coulomb interaction between nuclei and electrons will contract the radial wave function, which shrinks the ionic radius. On the other hand, the increasing unpaired spin expands the radial distribution of the electrons. These two aspects contribute to the monotonic increase of the 3D1 peak energy with the decreasing ionic radii shown in Fig. 2b. We especially highlight the highly covalent Ruddlesden-Popper cuprate La2CuO4 where the overlap between TM and La is further enhanced by reduced charge and intercalation of La in the rock-salt layer. We also note the fact that Co3+ lies on the turning point, indicating that the highest ionicity (lowest covalency) between La-TM might correlate with the optimal cathode potential in Li-ion batteries61.
a RIXS 3D1 energy and TM ionic radii as a function of nominal 3d electron counts. Note that the axis of the ionic radii is plotted inversely. b Negative correlation between RIXS 3D1 energy and TM (and Al3+) ionic radii in perovskite oxides and Ruddlesden-Popper La2CuO4. We define the error bars as three times the standard errors80 obtained from the model fits. Dashed linear regression line serves as a guide to the eye. High-spin ionic radii are selected for panel (b).
In the following, we use density functional theory (DFT) calculations of the element- and orbital-resolved density of states (DOS) in combination with our experimental measurements in order to thoroughly understand the different intra- and interatomic hybridizations at play in LaMO3.
Covalency is defined as the wavefunction overlap integral divided by the energy difference62,63. By constraining the geometric structure, coordination number and varying the 3d electron numbers, we compare both spatial-overlap-driven covalency (Experiment) and energetic-overlap-driven covalency (Theory). A larger spatial orbital overlap is generally associated with a higher hopping term, which leads to a broader band. The results of our approach are summarized in Fig. 3.
a Schematic view of the projected DOS. The elements, atomic orbitals, and their relative energy levels as well as distributions are illustrated. b–i Calculation results of LaMO3 (M = Ti-Ni respectively) and La2CuO4. La 4f (in blue) and La 5p (in orange) are highlighted in thicker line width and are used for the following band width analysis. TM denotes transition metal. The Fermi level is denoted as Ef.
From an energetic overlap perspective, our DFT calculations first show a clear energetic overlap of the La 4f with TM 3d from Ti to Mn (n = 1–4). Moving on to late TMs, the 4f band isolates from the 3d band. In addition, TM 3d orbitals hybridize with the O 2p orbitals, which further intra-atomically hybridize with O 2s, also known as O sp3 hybrid orbitals. At last, the O 2s and La 5p orbitals hybridize as they lie close to each other in terms of energies. We note that even though La RIXS at N4,5 edges does not directly probe the La 5d orbitals, intra-atomic 4f-5d hybridization was proposed earlier64,65. Therefore we cannot exclude the La 5d - La 4f mechanism from the RIXS chemical shift. From crystal field’s perspective, La 5d - O 2p interaction is expected to be larger than the TM 3d - O 2p due to the extended radial distribution. Likewise, we cannot exclude the La 5d - TM 3d (O 2p) - La 4f hybridization pathways.
Orbital hybridization modifies the effective on-site Coulomb repulsion U and hopping term t under Hubbard model. Hence, the hybridization is associated with the band width. To further address the degree of hybridization, we analyze the La 5p and 4f band widths (Wp and Wf, respectively), extracted from the projected density of states by taking the energy difference between the top and bottom edges of the La 5p and 4f peaks respectively. In particular, states below 2% of the peaks maxima are neglected. First, we plot the sum of the Wp and Wf against RIXS energy [Fig. 4a]. The trend in Wp + Wf along the 3d TM series is akin to the 3D1 RIXS energy. Figure 4b displays how the Wp and Wf evolve across the TM series: When going from early TM to late TM, the 4f band shrinks, indicative of a more localized character, while the La 5p band expands, indicative of a more itinerant character. This can be partially rationalized by the increasing effective nuclear charge of the TM. Heavier TM will shrink the effective ionic radius, which decreases the overlap with the La 4f band. Therefore, while in early TM perovskites the TM 3d band has moderate overlap with the La 4f band, the La 4f starts to localize when going to late TM. This trend can be linked back to the experimental observations: as the TM ionic radius decreases, the 3d orbital wave function has less overlap with the La 4f wave function. Finally, we examine the layered perovskite La2CuO4 which has an even higher orbital overlap and a larger ionic radius due to the nominal charge Cu2+ and Ruddlesden-Popper structure. The increase in band widths and RIXS red shift of La2CuO4 observed in Fig. 4 further indicate high La-Cu covalency besides the well-known Cu-O covalency. However, it is still unclear whether it is the structure change or the ionic radius change that has more contribution in the RIXS redshift.
a Trends of RIXS energy and bandwidth sum. The error bars are defined as three times the standard errors from the model fits. Note that the axis of the bandwidth sum in blue is plotted inversely. b Trends of the La 5p band widths (Wp) and the La 4f band widths (Wf) across the 3d transition metal perovskite oxide series and La2CuO4.
Conclusion
Our work demonstrates the direct observation of orbital-overlap-driven La-TM and La-O hybridization via high-resolution RIXS 3D1 final state. By increasing the orbital overlap between TM and La, we increase the band widths and reduce the energy distances of the La 5p-4f transition. The hybridization mechanism of the valence and conduction band is established between La (4f and 5p), TM (partially filled 3d) and O (2p and 2s). With help of DFT calculation, we observe that the 4f bands are getting more localized when increasing TM atomic number, whereas the 5p electrons are getting slightly more delocalized on the La atom in the LaMO3 series. Our investigation provides experimental evidence for the previously hypothesized role of covalency between La and the neighboring ions. The 4f covalency is contributed from hybridization with the TM 3d, whereas the 5p covalency is from the O 2s. Last, we further enhance the radial overlap between La and TM by probing highly covalent layered perovskite La2CuO4. An even larger redshift in RIXS 3D1 is achieved by reduced-charge-enhanced ionic radius and the intercalation of La atoms in rock-salt layers. The experimental result suggests that the La atom does not just serve solely as a spacer but electronically contributes to the band formation in perovskite oxides and the parent compound of cuprate superconductors.
Methods
The N4,5-edges RIXS experiments were performed at BESSY II (Helmholtz-Zentrum Berlin) at the beamline UE112-PGM1 with the meV-RIXS spectrometer66 and beamline U4967 with the SolidFlexRIXS end station68. All thin films were grown on SrTiO3 (STO) substrate if not explicitly noted. LaTiO3, LaVO369, LaCrO3, LaFeO3 (Nb:STO), LaCoO3 (on STO and LaAlO3 substrates)70,71, LaNiO3, and La2CuO4 (on LaSrAlO4 substrate)72 thin films were grown by pulsed laser deposition. LaMnO3 thin film was grown by molecular-beam epitaxy. Single crystal LaCoO3 was grown by the floating-zone method73. The samples were acquired from the laboratory sources and details about synthesis methods and characterizations can be found in the according references. The RIXS measurements were carried out at room temperature at 10−8 mbar pressure with a linearly horizontally polarized beam. The experimental geometry was defined with the incident beam at 30∘ to the sample surface at a fixed scattering angle of 90∘ on the horizontal plane. The combined spectral resolution is ≈ 60 meV. Each RIXS spectrum required at least 40 minutes data-acquisition time for sufficient statistical counts.
Calculations were performed using the projector augmented wave scheme as implemented in the VASP code74,75,76. Heyd-Scuseria-Ernzerhof screened hybrid functionals77 have been employed for LaMO3 with M = Ti-Co and La2CuO4. Different values of the mixing parameter have been chosen to better reproduce the experimental band gap. In particular, for LaMO3 we used the values reported in Ref. 41, while we chose α = 0.25 for La2CuO4. The correlated metal LaNiO3 has been described using the PBE functional78, as even small corrections open an unphysical band gap41. Low temperature, experimental structures41,79 are used for all compounds. k-point space is sampled with 4 × 4 × 4, 6 × 6 × 6 and 4 × 4 × 2 grids for M = Ti-Fe/M = Ni, M = Co and La2CuO4, respectively.
Data availability
Full RIXS datasets collected in this study are available from the corresponding author upon reasonable request.
References
Varignon, J., Grisolia, M. N., Íñiguez, J., Barthélémy, A. & Bibes, M. Complete phase diagram of rare-earth nickelates from first-principles. npj Quantum Mater. 2, 21 (2017).
Bisogni, V. et al. Ground-state oxygen holes and the metal-insulator transition in the negative charge-transfer rare-earth nickelates. Nat. Commun. 7, 13017 (2016).
Mercy, A., Bieder, J., Íñiguez, J. & Ghosez, P. Structurally triggered metal-insulator transition in rare-earth nickelates. Nat. Commun. 8, 1677 (2017).
Hepting, M. et al. Tunable charge and spin order in prnio3 thin films and superlattices. Phys. Rev. Lett. 113, 227206 (2014).
Pavarini, E. & Koch, E. Origin of jahn-teller distortion and orbital order in LaMnO3. Phys. Rev. Lett. 104, 086402 (2010).
Bhattacharya, D., Devi, P. S. & Maiti, H. S. Orbital order-disorder transition in La1−x NdxMnO3 (x = 0.0-1.0) and La1−x−yNdxSryMnO3 (x = 0.1; y = 0.05,0.1). Phys. Rev. B 70, 184415 (2004).
Okamoto, S., Ishihara, S. & Maekawa, S. Phase transition in perovskite manganites with orbital degree of freedom. Phys. Rev. B 61, 14647–14655 (2000).
Bednorz, J. G. & Müller, K. A. Perovskite-type oxides—the new approach to high-Tc superconductivity. Rev. Mod. Phys. 60, 585–600 (1988).
Keimer, B., Kivelson, S. A., Norman, M. R., Uchida, S. & Zaanen, J. From quantum matter to high-temperature superconductivity in copper oxides. Nature 518, 179–186 (2015).
Li, D. et al. Superconductivity in an infinite-layer nickelate. Nature 572, 624–627 (2019).
Zhu, H., Zhang, P. & Dai, S. Recent advances of lanthanum-based perovskite oxides for catalysis. ACS Catal. 5, 6370–6385 (2015).
Baeumer, C. et al. Tuning electrochemically driven surface transformation in atomically flat LaNiO3 thin films for enhanced water electrolysis. Nat. Mater. 20, 674–682 (2021).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Kostopoulou, A., Kymakis, E. & Stratakis, E. Perovskite nanostructures for photovoltaic and energy storage devices. J. Mater. Chem. A 6, 9765–9798 (2018).
Hu, S. et al. Selected multiferroic perovskite oxides containing rare earth and transition metal elements. Chinese Sci. Bull. 59, 5170–5179 (2014).
Lone, I. H. et al. Multiferroic ABO3 transition metal oxides: a rare interaction of ferroelectricity and magnetism. Nanoscale Res. Lett. 14, 142 (2019).
Goodenough, J. B. Theory of the role of covalence in the perovskite-type manganites [La, M(II)]MnO3. Phys. Rev. 100, 564–573 (1955).
Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 10, 87–98 (1959).
Bouarab, S., Vega, A. & Khan, M. A. Ab initio optical conductivity in LaMO3 (M = Ti-Cu). Phys. Rev. B 54, 11271–11275 (1996).
Kim, B., Liu, P., Tomczak, J. M. & Franchini, C. Strain-induced tuning of the electronic coulomb interaction in 3d transition metal oxide perovskites. Phys. Rev. B 98, 075130 (2018).
Frati, F., Hunault, M. O. & De Groot, F. M. Oxygen K-edge X-ray absorption spectra. Chem. Rev. 120, 4056–4110 (2020).
Alonso, J. A., Martínez-Lope, M. J., Casais, M. T., García-Muñoz, J. L. & Fernández-Díaz, M. T. Room-temperature monoclinic distortion due to charge disproportionation in RNiO3 perovskites with small rare-earth cations (R = Ho, Y, Er, Tm, Yb, and Lu): a neutron diffraction study. Phys. Rev. B 61, 1756–1763 (2000).
Prosandeev, S., Bellaiche, L. & Íñiguez, J. Ab initio study of the factors affecting the ground state of rare-earth nickelates. Phys. Rev. B 85, 214431 (2012).
Kim, M. W. et al. Effect of orbital rotation and mixing on the optical properties of orthorhombic RMnO3 (R = La, Pr, Nd, Gd, and Tb). Phys. Rev. Lett. 96, 247205 (2006).
Petit, L., Svane, A., Szotek, Z. & Temmerman, W. M. First-principles study of rare-earth oxides. Phys. Rev. B 72, 205118 (2005).
El-Kelany, K. E. et al. Spin localization, magnetic ordering, and electronic properties of strongly correlated \({{{{{{{{\rm{Ln}}}}}}}}}_{2}{{{{{{{{\rm{O}}}}}}}}}_{3}\) sesquioxides (Ln = La, Ce, Pr, Nd). Phys. Rev. B 97, 245118 (2018).
Hepting, M. et al. Electronic structure of the parent compound of superconducting infinite-layer nickelates. Nat. Mater. 19, 381–385 (2020).
Löble, M. W. et al. Covalency in Lanthanides. An X-ray Absorption Spectroscopy and Density Functional Theory Study of \({{{{{{{{{\rm{LnCl}}}}}}}}}_{6}}^{x-}\) (x = 3, 2). J. Am. Chem. Soc. 137, 2506–2523 (2015).
Peng, C., Jiang, H.-C., Moritz, B., Devereaux, T. P. & Jia, C. Charge order and superconductivity in a two-band model for infinite-layer nickelates. Phys. Rev. B 108, 245115 (2023).
Tam, C. C. et al. Charge density waves in infinite-layer NdNiO2 nickelates. Nat. Mater. 21, 1116–1120 (2022).
Jiang, P., Si, L., Liao, Z. & Zhong, Z. Electronic structure of rare-earth infinite-layer RNiO2(R = La, Nd). Phys. Rev. B 100, 201106 (2019).
Zhang, R. et al. Magnetic and f-electron effects in LaNiO2 and NdNiO2 nickelates with cuprate-like 3d\({}_{{x}^{2}-{y}^{2}}\) band. Commun. Phys. 4, 118 (2021).
Pan, G. A. et al. Superconductivity in a quintuple-layer square-planar nickelate. Nat. Mater. 21, 160–164 (2022).
Liu, T. et al. Dimensionality-induced insulator-metal crossover in layered nickelates Lan+1NinO2n+2 (n = 2, 3, and ∞). AIP Adv. 4 047132 (2014).
Wang, L. et al. Tuning bifunctional oxygen electrocatalysts by changing the a-site rare-earth element in perovskite nickelates. Adv. Funct. Mater. 28, 1803712 (2018).
Been, E. et al. Electronic structure trends across the rare-earth series in superconducting infinite-layer nickelates. Phys. Rev. X 11, 011050 (2021).
Lee, K.-W. & Pickett, W. E. Infinite-layer LaNiO2: Ni1+ is not Cu2+. Phys. Rev. B 70, 165109 (2004).
Gu, Y., Zhu, S., Wang, X., Hu, J. & Chen, H. A substantial hybridization between correlated Ni-d orbital and itinerant electrons in infinite-layer nickelates. Commun. Phys. 3, 84 (2020).
Higashi, K., Winder, M., Kuneš, J. & Hariki, A. Core-level x-ray spectroscopy of infinite-layer nickelate: LDA + DMFT study. Phys. Rev. X 11, 041009 (2021).
Pari, G., Mathi Jaya, S., Subramoniam, G. & Asokamani, R. Density-functional description of the electronic structure of LaMO3 (M = Sc,Ti,V,Cr,Mn,Fe,Co,Ni). Phys. Rev. B 51, 16575–16581 (1995).
He, J. & Franchini, C. Screened hybrid functional applied to 3d0 → 3d8 transition-metal perovskites LaMO3 (M = Sc–Cu): Influence of the exchange mixing parameter on the structural, electronic, and magnetic properties. Phys. Rev. B 86, 235117 (2012).
May, K. J. & Kolpak, A. M. Improved description of perovskite oxide crystal structure and electronic properties using self-consistent Hubbard U corrections from ACBN0. Phys. Rev. B 101, 165117 (2020).
Franchini, C. Hybrid functionals applied to perovskites. J. Phys. Condens. Matter 26, 253202 (2014).
Varrassi, L. et al. Optical and excitonic properties of transition metal oxide perovskites by the bethe-salpeter equation. Phys. Rev. Mater. 5, 074601 (2021).
Ergönenc, Z., Kim, B., Liu, P., Kresse, G. & Franchini, C. Converged GW quasiparticle energies for transition metal oxide perovskites. Phys. Rev. Mater. 2, 024601 (2018).
Zaanen, J., Sawatzky, G. A. & Allen, J. W. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 55, 418–421 (1985).
Suntivich, J. et al. Estimating Hybridization of Transition Metal and Oxygen States in Perovskites from O K-edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 118, 1856–1863 (2014).
de Groot, F. M. F. et al. Oxygen 1s x-ray-absorption edges of transition-metal oxides. Phys. Rev. B 40, 5715–5723 (1989).
Walters, A. C. et al. Effect of covalent bonding on magnetism and the missing neutron intensity in copper oxide compounds. Nat. Phys. 5, 867–872 (2009).
Ament, L. J., Van Veenendaal, M., Devereaux, T. P., Hill, J. P. & Van Den Brink, J. Resonant inelastic x-ray scattering studies of elementary excitations. Rev. Mod. Phys. 83, 705–767 (2011).
Gilmore, K. et al. Description of resonant inelastic x-ray scattering in correlated metals. Phys. Rev. X 11, 031013 (2021).
Mitrano, M. & Wang, Y. Probing light-driven quantum materials with ultrafast resonant inelastic x-ray scattering. Commun. Phys. 3, 184 (2020).
Huang, H. Y. et al. Resonant inelastic X-ray scattering as a probe of Jeff = 1/2 state in 3d transition-metal oxide. npj Quantum Mater. 7, 33 (2022).
Kotani, A. & Shin, S. Resonant inelastic x-ray scattering spectra for electrons in solids. Rev. Mod. Phys. 73, 203–246 (2001).
Schülke, W. Electron Dynamics by Inelastic X-Ray Scattering 7 edn. (Oxford University Press, 2007).
Liu, C.-Y. et al. Role of La 5p in bulk and quantum-confined solids probed by the La 5p54f13D1 excitonic final state of resonant inelastic X-ray scattering. J. Phys. Chem. C 127, 11111–11118 (2023).
Suljoti, E. et al. Spin-Orbit mediated interference in the radiative and nonradiative channels of the La 4d core resonances. Phys. Rev. Lett. 103, 137401 (2009).
Moewes, A., Postnikov, A. V., Kurmaev, E. Z., Grush, M. M. & Ederer, D. L. Resonant mixing of widely separated intermediate states and charge transfer at the 4d-4f resonance of La compounds. Europhys. Lett. 49, 665–671 (2000).
Epstein, G. L. & Reader, J. Spectrum and energy levels of triply ionized lanthanum (La iv). J. Opt. Soc. Am. 69, 511 (1979).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Vitova, T., Roesky, P. W. & Dehnen, S. Open questions on bonding involving lanthanide atoms. Commun. Chem. 5, 12 (2022).
Lu, E. et al. Emergence of the structure-directing role of f-orbital overlap-driven covalency. Nat. Commun. 10, 634 (2019).
Zhang, L. et al. Strong-correlated behavior of 4f electrons and 4f5d hybridization in PrO2. Sci. Rep. 8, 1–8 (2018).
Nohara, Y., Yamamoto, S. & Fujiwara, T. Electronic structure of perovskite-type transition metal oxides LaMO3(M = Ti ~ Cu) by U + GW approximation. Phys. Rev. B 79, 195110 (2009).
Bauer, K. et al. The meV XUV-RIXS facility at UE112-PGM1 of BESSY II. J. Synchrotron Radiat. 29, 908–915 (2022).
Kachel, T. The plane grating monochromator beamline U49-2 PGM-1 at BESSY II. J. Large-scale Res. Facil. JLSRF 2, A72 (2016).
Beye, M. & Miedema, P. S. Solid flexRIXS: A RIXS endstation for solid systems at BESSY II. J. Large-scale Res. Facil. JLSRF 3, A124 (2017).
Ruotsalainen, K. et al. Resonant inelastic x-ray scattering study of doping and temperature dependence of low-energy excitations in La1−xSrxVO3 thin films. Phys. Rev. B 103, 235158 (2021).
Wang, R.-P. et al. Low-energy orbital excitations in strained LaCoO3 films. Phys. Rev. B 100, 165148 (2019).
Hariki, A. et al. Damping of spinful excitons in LaCoO3 by thermal fluctuations: Theory and experiment. Phys. Rev. B 101, 245162 (2020).
Martinelli, L. et al. Collective Nature of Orbital Excitations in Layered Cuprates in the Absence of Apical Oxygens. Phys. Rev. Lett. 132, 066004 (2024).
Tomiyasu, K. et al. Coulomb Correlations Intertwined with Spin and Orbital Excitations in LaCoO3. Phys. Rev. Lett. 119, 196402 (2017).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Jorgensen, J. D. et al. Superconducting phase of La2CuO4+δ: A superconducting composition resulting from phase separation. Phys. Rev. B 38, 11337–11345 (1988).
Ivashko, O. et al. Strain-engineering mott-insulating La2CuO4. Nat. Commun. 10, 786 (2019).
Acknowledgements
We are grateful for the awarded beamtimes at BESSY II operated by the Helmholtz-Zentrum Berlin für Materialien und Energie. K.S. thanks the Humboldt Foundation for funding from the Humboldt Research Fellowship for postdoctoral researchers program. L.C. acknowledges the Vienna Doctoral School of Physics. The computational results have been achieved using the Vienna Scientific Cluster (VSC). M.L. and F.d.G. received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860553.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.-Y.L. and A.F. conceptualized the project. L.C. performed theoretical calculations. C.-Y.L., K.R., K.S., M.K., R.D., K.N.P., and M.L. planned and performed the experiments. R-P.W., I.I.O., D.J.K., D.D.C., K.T., E.M., Y.A.B., E.M.K., I.C.G.B., and M.L. prepared and synthesized the samples. C.B., G.K. and C.D. supervised the PLD/MBE synthesis and laboratory-based characterization methods. C.-Y.L. analyzed the experimental data. F.d.G., C.F. supervised theoretical interpretation. C.-Y.L., L.C., R.D., K.R., Y.A.B., C.B. and A.F. wrote the manuscript with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Physics thanks Clemens Ulrich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, CY., Celiberti, L., Decker, R. et al. Orbital-overlap-driven hybridization in 3d-transition metal perovskite oxides LaMO3 (M = Ti-Ni) and La2CuO4. Commun Phys 7, 156 (2024). https://doi.org/10.1038/s42005-024-01642-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42005-024-01642-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.