Abstract
The Wnt-planar cell polarity (Wnt-PCP) pathway is crucial in establishing cell polarity during development and tissue homoeostasis. This pathway is found to be dysregulated in many pathological conditions, including cancer and autoimmune disorders. The central event in Wnt-PCP pathway is the activation of Weak-similarity guanine nucleotide exchange factor (WGEF) by the adapter protein Dishevelled (Dvl). The PDZ domain of Dishevelled2 (Dvl2PDZ) binds and activates WGEF by releasing it from its autoinhibitory state. However, the actual Dvl2PDZ binding site of WGEF and the consequent activation mechanism of the GEF have remained elusive. Using biochemical and molecular dynamics studies, we show that a unique “internal-PDZ binding motif” (IPM) of WGEF mediates the WGEF-Dvl2PDZ interaction to activate the GEF. The residues at P2, P0, P-2 and P-3 positions of IPM play an important role in stabilizing the WGEFpep-Dvl2PDZ interaction. Furthermore, MD simulations of modelled Dvl2PDZ-WGEFIPM peptide complexes suggest that WGEF-Dvl2PDZ interaction may differ from the reported Dvl2PDZ-IPM interactions. Additionally, the apo structure of human Dvl2PDZ shows conformational dynamics different from its IPM peptide bound state, suggesting an induced fit mechanism for the Dvl2PDZ-peptide interaction. The current study provides a model for Dvl2 induced activation of WGEF.
Similar content being viewed by others
Introduction
Wnt signalling pathways regulate diverse cellular functions during embryonic development and tissue homoeostasis1. The central event in Wnt pathways involves activation of hepta helical membrane receptors, Frizzleds, by the secreted glycoproteins, Wnts. The signalling further propagates on the subsequent recruitment of a scaffold protein, Dishevelled (Dvl), by the C-terminal target binding domain of the Frizzled receptors. This signal further diverges into two modes; canonical β-catenin-dependent signalling and non-canonical β-catenin-independent signalling. The canonical β-catenin-dependent signalling promotes the expression of target genes by facilitating the nuclear translocation of β-catenin and subsequent activation of TCF/LEF transcription factors associated with those target genes. This mode of Wnt signalling is majorly involved in cellular processes like cell proliferation2. On the other hand, the non-canonical β-catenin-independent signalling is further subdivided into Wnt/Calcium and Wnt-planar cell polarity (Wnt-PCP) pathways3,4. These non-canonical pathways are shown to control cell polarity and migration2. Aberrations in the Wnt signalling pathways are implicated in many pathological conditions, including cancer, developmental and autoimmune disorders5,6.
One of the key features of Wnt-PCP pathway is the regulation of actin cytoskeleton reorganization through the activation of Rho GTPases, namely RhoA, Rac and Cdc427. Rho GTPases function as molecular switches that cycle between GTP bound ON (active) and the GDP bound OFF (inactive) states8. This switching between ON and OFF states is brought about by the regulatory proteins known as Guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), respectively9. Appropriate signalling cues, transducing from the membrane receptors such as G-protein coupled receptors (GPCRs)10, receptor tyrosine kinase11 and integrin receptors12, activate their downstream GEFs, which in turn trigger Rho GTPases by exchanging their bound GDP with GTP. However, the majority of GEFs exist in an autoinhibitory state and require other signalling proteins for their activation10. In the context of Wnt-PCP pathway WGEF (Weakly similar to RhoGEF 5/TIM), also known as Arhgef19/ Ephexin2), activates RhoA13,14. In Xenopus, WGEF-RhoA exerts convergent extension during gastrulation. Thus, marking the Wnt-PCP/RhoA pathway as one of the critical pathways involved in developmental and tissue regeneration processes in the higher eukaryotes15,16,17,18. Similar to canonical DH-PH GEFs, like TIM and Ephexin, WGEF consists of the conserved catalytic Dbl homology (DH) domain responsible for its GEF activity and the Pleckstrin homology (PH) domain, which is involved in the membrane localization of the GEF13. Furthermore, WGEF consists of a C-terminal Src homology 3 (SH3)13 and an N-terminal inhibitory (NID) domain. It has been shown that the phosphorylation of a conserved tyrosine in the NID region is critical in the activation of the GEF19,20,21. Besides NID, the SH3 domain also contributes to the autoinhibition of the GEF through its intra- and intermolecular interactions21,22,23.
In the context of the Wnt-PCP pathway, the adapter protein Dishevelled is shown to be involved in releasing the autoinhibitory state of WGEF17. Dishevelled (Dvl), which has three paralogs in humans, viz., Dvl1, Dvl2 and Dvl3, operate as a signalling hub in exerting both canonical and non-canonical Wnt signalling pathways24,25. This protein consists of three conserved domains, namely, an N-terminal DIX (Dishevelled-Axin) domain, a central PDZ (PSD-95, DLG, ZO1) domain and a C-terminal DEP (Dishevelled, EGL-10, Pleckstrin) domain26. These domains play diverse roles during the Wnt signalling pathways27,28,29,30. Regarding the activation of WGEF, the PDZ domain of Dishevelled2 (Dvl2) is shown to be the key player. Dvl2 binds to WGEF through its PDZ domain to release the GEF from its autoinhibitory state17. However, the exact region of WGEF that interacts with Dvl2PDZ and its mode of interaction with Dvl2 have remained elusive. In the present study, we have identified an evolutionary conserved Dvl2PDZ binding motif in WGEF (hereafter referred to as WGEFpep), which facilitates the activation of the GEF by disrupting its autoinhibitory state. Using biochemical assays and molecular dynamics simulation studies, we have elucidated the mode of Dvl2PDZ–WGEF interaction and its consequence on the activation of the GEF.
Results
A novel N-terminal conserved ‘internal peptide’ motif of WGEF mediates its interaction with Dvl2PDZ
Previously, Igor et al. have shown that the PDZ domain of Dvl2 binds to the N-terminal domain of WGEF, resulting in activation of the GEF. Further, deletion of the first 213 residues from the N-terminal of human WGEF (hWGEFΔ213) has been shown to reduce its affinity for Dvl2 considerably but at the same time to significantly enhance its GEF activity. Thus, it was suggested that the N213 region of the GEF could be involved in WGEF–Dvl2PDZ interaction17. In another study, hWGEFΔ302N-term mutant is shown to be free from autoinhibition, suggesting that its PDZ binding motif precedes the autoinhibitory domain of the GEF (spanning residues 291-NSVLYQEY-298)17,21. However, the exact motif of WGEF that binds to Dvl2PDZ is unclear from these studies. Like other PDZ domains, the Dvl2PDZ also exhibits promiscuity in terms of the peptide motifs that it binds to. The peptide motifs that PDZ domains recognize are primarily C-terminal peptide motifs, which are composed of a stretch of 7–10 residues situated at the C-terminus of the binding partners. Apart from these, some PDZ domains can also recognize the internal motifs that possess a similar number of residues but can lie anywhere in their binding partners31,32,33. Binding of PDZ with internal motifs mimic that of the C-terminal peptide binding with slight variations in the residues of the peptide involved in the binding33,34.
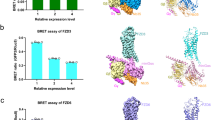
Although not very stringent, there is some consensus in the sequences of C-terminal peptide motifs that bind to PDZ domains31,32. However, there is no statistically significant data to arrive at a consensus sequence for the PDZ domain binding internal peptide motifs. Therefore, to identify the Dvl2PDZ interacting internal motif of WGEF, we searched the sequences of this family of GEFs with the putative Dvl2PDZ binding motifs identified from peptide-phage display studies35. We employed three prominent peptide motifs, X-Y-G-W-Φa-D/G, X-W-Φa-D-G-P and W-Φs-D-X-P (where X, Φa and Φs are any, aliphatic and short side chain hydrophilic amino acids [S/T], respectively), as templates for the search. The only hit, 349-GSTFSLWQDIP-359 (hereafter referred to as WGEFpep), obtained from the sequence search, lies between the DH and the inhibitory domain of hWGEF (Fig. 1a). This particular motif is highly conserved amongst WGEFs family, indicating it to be the most putative Dvl2PDZ binding site present in WGEFs (Fig. 1a and Supplementary Fig. 1). This observation is contrary to the previous study in which the Dvl2PDZ binding was mapped towards the N-terminus of hWGEF (between amino acid residues 1–213)17, whereas the motif identified by us is present between amino acid residues 349–359 of hWGEF. Hence, to validate our observations, we designed constructs of the GEF comprising both the autoinhibitory and the Dvl2PDZ binding motif and yet excluding the major portion of its N-terminus. Since some of these hWGEF constructs were found to be insoluble and/or poorly expressed in E. coli, we used WGEF from Xenopus laevis (xWGEF) as a surrogate system for further studies. To test the influence of Dvl2PDZ binding on the catalytic activity of WGEFs, we performed nucleotide exchange assays on these constructs, both in the presence and absence of the PDZ domain. Clearly, the xWGEF330 (equivalent to hWGEF272) construct, which is composed of both the inhibitory and the PDZ binding domains, showed insignificant GEF activity. However, in the presence of 10-fold excess (50 µM) of Dvl2PDZ, there is a fourfold increase in its GEF activity (Fig. 1b, c). It is worth noting that the lower concentration of Dvl2PDZ (25 µM) did not show any observable change in the GEF activity. These observations suggest that the binding of Dvl2PDZ indeed promotes the activity of GEF; however, the affinity of the xWGEF330 for Dvl2PDZ is considerably low (Fig. 1b, c). Next, to show that the identified internal peptide motif of WGEF, 402(349)-GSTFSLWQDIP-412(359)/ WGEFpep [residue numbers shown in the parentheses corresponds to hWGEF], binds to Dvl2PDZ, we performed the GEF assays in the presence of both Dvl2PDZ and WGEFpep. Since the internal peptides are shown to have relatively low affinity for PDZ domains, we used a fourfold molar excess of WGEFpep (200 µM) to disrupt the WGEF–Dvl2PDZ interaction (Fig. 1b, c). From this exercise, it is clear that WGEFpep competitively binds to the PDZ domain and replaces the WGEF in Dvl2PDZ–WGEF interaction, causing a decrease in the catalytic activity of WGEF. Thus, our observations unambiguously suggest that the 402–412 region of xWGEF is the ‘internal peptide motif’ that mediates its interactions with Dvl2PDZ.
a Schematic representation of WGEF domains (N-terminal Inhibitory: NID, Dbl homology: DH, pleckstrin-homology: PH and src Homology-3: SH3) and sequence alignment of the conserved PDZ binding motif of Zebrafish (Uniprot ID: E7EY470), Xenopus (Uniprot ID: A5X5J0), Mouse (Uniprot ID: Q8BWA8), Human (Uniprot ID: Q8IW93-1) and Bovine (Uniprot ID: E1BQ24) ahead of DH domain in WGEF. The amino acid residue numbers in the upper and lower parts of the diagram correspond to hWGEF and xWGEF, respectively. b GEF assay plots of xWGEF330, xWGEF330L407A and xWGEF330Y353E, in the presence and absence of Dvl2PDZ and peptide variants c Bar graph showing relative GEF activities of xWGEF variants normalized against that of wild-type (autoinhibited xWGEF330) protein. The error bar and * represent standard deviation (±SD) and significance as ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P ≤ 0.01, respectively. d Sequence alignment of Dvl2PDZ binding internal peptides and WGEF internal peptide sequence. Residue positions are labelled in red.
WGEFpep motif–Dvl2PDZ interaction is sufficient to partially release the autoinhibition of WGEF
Canonically, PDZ domains bind to C-terminal peptides, and therefore, the residue at the extreme C-terminal of the peptide is referred to as the 0th or P0 residue. The remaining residues towards the N-terminus of the peptide are numbered in reverse order as ‘−1’, ‘−2’, ‘−3’ or P−1, P−2, P−3, and so on36,37,38. Dvl2PDZ binds to both C-terminal as well as the non-canonical internal peptides35,39. Therefore, we will refer to the residue that interacts with the X-Φ-G-Φ motif of the carboxylate binding loop (X represents any amino acid and Φ represents hydrophobic residues)31 as P0, followed by P−1, P−2, P−3 for residues preceding P0 and P1, P2, P3 for the residues that lie towards the C-terminus.
Zhang et al. have reported earlier that the side chain of aspartate residue present in the internal peptide mimics the free C-terminal carboxylate group and binds to the carboxylate binding loop of Dvl2PDZ. Thus, this particular interaction is asserted to stabilize the Dvl2PDZ-effector binding35. Therefore, to test whether the WGEFpep follows this “usual” internal peptide–Dvl2PDZ interaction, we substituted the aspartate present at the P0th (Fig. 1d) position of the peptide with alanine (WGEFpepD9A). Interestingly, with an excess amount of WGEFpepD9A peptide, there was a decrease in the exchange activity of WGEF, but not to an extent brought out by the wild-type WGEFpep (Fig. 1b, c). We performed a comparable exercise on GEF by replacing this particular aspartate (xWGEF330D410A) in xWGEF construct. Since this xWGEF mutant was found to be insoluble, no further studies could be conducted. Other studies also suggest that proline, tryptophan and leucine at P2, P−2 and P−3, positions, respectively, also play a key role in stabilizing Dvl2PDZ–effectors interactions35. To test the role of these residues when they are part of the GEF, we produced xWGEF330P412A, xWGEF330W408A and xWGEF330L407A mutants; however, only the xWGEF330L407A was found to be soluble. Surprisingly, the enhanced GEF activity of xWGEF330L407A is independent of its interaction with Dvl2PDZ (50 µM) (Fig. 1b, c), as the addition of Dvl2PDZ (50 μM) does not seem to affect its GEF activity. The higher GEF activity observed for xWGEF330L407A could be due to the structural rearrangement caused by the mutation. However, from our experiments it is not clear how L407A substitution influences the autoinhibition of the GEF. Thus, this aspect requires further investigations.
Next, to investigate whether the WGEF–Dvl2PDZ interaction promotes the GEF activity by releasing its autoinhibition or it follows some unknown activation mechanism, we generated a Y353E mutant of xWGEF330. This particular tyrosine (Y353xWGEF/295hWGEF) of hWGEF is present in NID and locks the GEF by interacting with its DH domain. Furthermore, hWGEFY295E is shown to exist in autoinhibition-free form21. Interestingly, the xWGEF330Y353E mutant exhibited a nearly threefold increase in the GEF activity compared to its wild-type form. Further, the presence of Dvl2PDZ (50 µM) did not alter the GEF activity of the mutant (Fig. 1b, c). Thus, these results suggest that the Dvl2PDZ binds to the WGEFpep region of the GEF, lying between its inhibitory (NID) and the DH domains. Furthermore, this particular interaction between WGEF and Dvl2PDZ activates the GEF by partially releasing it from its autoinhibitory state regulated by the NID. The mechanism through which the autoinhibition governed by the SH3 domain is released remains unknown.
Binding of WGEFpep with Dvl2PDZ is mediated by the residues at the P −2, P 0 and P 2 positions of the binding motif
Although the WGEFpep region is highly conserved in the WGEF family (Supplementary Fig. 1), the interaction between WGEF and Dvl2PDZ appears to be feeble. This aligns with the observations made for other PDZ–internal peptide interactions33,35. Perhaps differential affinity of the Dvl with its effectors is one of the strategies adopted by Wnt-Frizzled pathways to exert diverse signalling modes25,30. Although our GEF assay-based studies have shown that, indeed, the residue at the P0 position of WGEFpep plays a crucial role in mediating WGEF–Dvl2PDZ binding, it is not clear how variations in other residue positions influence the interaction. Therefore, to obtain the structural basis of Dvl2PDZ–WGEF interaction, initially, we tried to crystallize this protein complex. Despite extensive efforts, crystallization trials met with no success. Hence, we resorted to crystallizing the WGEFpep (peptide) in complex with Dvl2PDZ. However, even this attempt did not yield the desired result, as there was no electron density for the bound peptide in the crystal structure of the WGEFpep–Dvl2PDZ complex (PDB ID: 8WWR) (Table 1). Furthermore, we tried to obtain the WGEFpep–Dvl2PDZ complex structure by fusing the peptide with the C-terminus of the PDZ domain. Even this approach failed to produce the WGEFpep–Dvl2PDZ complex structure (PDB ID: 8YR7) (Supplementary Fig. 2 and Table 1). Therefore, we systematically designed divergent peptides of WGEFpep and biochemically probed their interaction with Dvl2PDZ. The closest homologue of WGEFpep is an engineered peptide, N3pep, whose structure in complex with Dvl2PDZ is available (PDB ID: 3CC0). Hence, by using this structure as template, we designed WGEFpep variants by successively substituting its residues from P−3 to P2 positions with alanine (Fig. 1d), as these residues are seen to be lying within 5 Å from Dvl2PDZ residues and they are conserved between WGEFpep and N3pep. To assess the differential contributions from these residues of the peptide, we determined the dissociation constants of the WGEFpep variants for Dvl2PDZ, employing Microscale Thermophoresis (MST). In addition to the WGEFpep variant peptides the minimal hWGEF (330–581) construct, obtained from our solubility screening, was used for the MST study (Fig. 2a, b, d and Supplementary Fig. 3). It is interesting to note that the affinities of WGEFpep and N3pep are nearly identical for Dvl2PDZ, with dissociation constants (Kd) of 45 ± 5 and 54 ± 7 µM, respectively (Fig. 2a, d and Table 2). This further validates the choice of our model for delineating WGEF–Dvl2PDZ interaction. Substitution of Gln at the P−1 (Fig. 1d) with Ala (WGEFpepQ8A) enhances the affinity of the peptide by almost 6-fold, whereas Ile at P1 to Ala (WGEFpepI10A) does not seem to affect the WGEFpep–Dvl2PDZ interaction (Fig. 2b, d and Table 2). It is interesting to note that amongst these two residues, only the side chain of Ser at P−1 makes direct interaction with Dvl2PDZ in the Dvl2PDZ–N3pep complex crystal structure. On the contrary, the substitution of Leu at the P−3 position (WGEFpepL6A) significantly reduces the affinity of the peptide for Dvl2PDZ (Fig. 2c, d and Table 2). Furthermore, the substitution of tryptophan (WGEFpepW7A), aspartate (WGEFpepD9A) and proline (WGEFpepP11A) residues at the P−2, P0 and P2 positions, respectively, with alanine completely abolishes the WGEF–Dvl2PDZ interactions (Table 2 and Supplementary Table 1). Thus, MST studies suggest that the residues at P−2, P0 and P2 positions (W, D & P) are crucial for the WGEF-Dvl2PDZ binding, whereas leucine at the P−3 position may influence the peptide–Dvl2PDZ interaction either by stabilizing the complex or by enhancing the solubility of the peptide.
a Binding curve corresponding to the interaction of Dvl2PDZ with WGEFpep and N3pep. b and c Binding curve corresponding to WGEFpepQ8A, WGEFpepI10A peptides, hWGEF330-581, and WGEFpepL6A peptide, respectively. d Bar plots showing dissociation constants between Dvl2PDZ and different peptides. The error bars represent Kd confidence obtained from triplicates.
Internal peptide ligands of Dvl2PDZ exhibit divergent modes of interaction
From GEF activity and MST studies, we have identified the residues of the internal peptide motif that drive the WGEF–Dvl2PDZ interaction. Furthermore, in both canonical and non-canonical (internal) peptides, the ligand adopts antiparallel β strand conformation and forms a β sheet by associating with the β2 strand of the PDZ domain40. Additionally, in the canonical binding mode, the C-terminus of the P0 residue engages with the X-Φ-G-Φ motif of the carboxylate binding loop to stabilize the complex (Fig. 1a, d). However, in the non-canonical PDZ domain binding, the carboxylate binding loop may not adopt a closed conformation and instead engages with the internal peptide through a network of hydrogen bonds involving either the main chain or the side chain atoms of the peptide35,37,41. Due to the lack of structural information on the WGEFpep–Dvl2PDZ complex, their binding mode has remained unclear. Hence, to address this aspect, we performed a total of 9 μs molecular dynamics simulations of the Dvl2PDZ structure, complexed with WGEFpep, WGEFpepQ8A and N3pep peptides. Since for two of the former peptides, there was no crystal structure available, we modelled the respective Dvl2PDZ–peptide complexes using the coordinates of the apo (PDB ID: 8WWR) (Table 1) and Dvl2PDZ-N3pep structures.
To understand the role of every residue in stabilizing the interaction, we plotted residue-wise peptide–PDZ domain interaction propensity (probability of the residue side-chain lying within 4 Å from the PDZ residue) (Fig. 3a–d). Propensity and the residue RMSF (Supplementary Fig. 4) plots obtained from MD simulations show that residues at positions P−2, P−1, P0, P1 and P2 play significant roles in stabilizing the peptide–Dvl2PDZ interaction. Amongst these, the aspartate residue of the P0 position of both WGEFpep and N3pep forms maximum number of interactions with X-Φ-G-Φ motif of the carboxylate binding loop (Fig. 3a, b, e, f). Apart from Asp at the P0 position, it was observed that residues at the positions P−1, P−2 and P−3 also make interactions with the residues belonging to the β2-α2 region of Dvl2PDZ (Fig. 3a, b, e, f). Interestingly, most interactions between WGEFpep-Dvl2PDZ are similar to those observed in the Dvl2PDZ-N3pep (Fig. 3a, b). However, subtle differences in the WGEFpep residues at P-4 and P-5 exhibit divergence in their interaction with the PDZ domain, when compared with those of N3pep interactions. In Dvl2PDZ–N3pep complex residues at P−4 and P−5 positions interact with β2 strand of Dvl2PDZ domain, which is absent in WGEFpep-Dvl2PDZ simulated model (Fig. 3a, b, e, f). Perhaps that is why in WGEFpep-Dvl2PDZ simulations, residues from P−4 onwards (towards N-terminus) exhibit higher RMSF values (Supplementary Fig. 4). Furthermore, the tryptophan at the P−2 position of N3pep provides additional stacking interactions to stabilize the PDZ–peptide complex.
a Interaction propensity with WGEFpep. b Interaction propensity with N3pep and c interaction propensity with WGEFpepQ8A. d Shows difference in the interaction propensity of WGEFpepQ8A and WGEFpep. e–g show the structure of Dvl2PDZ (represented as surface) bound with peptide variants, WGEFpep, N3pep and WGEFpepQ8A (represented in ball-and–sticks), belonging to one of the stable trajectories, respectively. h Superimposition of WGEFpep (yellow peptide) and WGEFpepQ8A (green peptide) structures.
Our MST studies show that the substitution of Gln at P−1 with Ala (WGEFpepQ8A) in WGEFpep considerably increases the affinity of the mutant peptide towards Dvl2PDZ by 6-folds (Fig. 2b, d). Interestingly, the MD simulations on the WGEFpepQ8A–Dvl2PDZ complex indicate a higher propensity for the Trp at the P−2 position to stay in the hydrophobic groove formed by Ile 280, Leu 278, Leu 337, and Val 341 residues at the β2-α2 region of the PDZ domain (Fig. 3c, d, g). However, the backbone conformation of this residue that favours the hydrophobic interaction with the PDZ domain is governed by the residue at the P−1 position. The presence of a residue with a longer side chain at P−1 could restrict the conformational space of Trp at P−2 and hence, may hinder the additional hydrophobic interactions between this residue and the PDZ domain (Fig. 3h). Thus, substitution of Gln with a shorter-side chain amino acid, like Ala, at P−1 position promotes WGEFpep–Dvl2PDZ interaction, as seen from MST studies. Thus, our MD studies provide insights into the Dvl2PDZ–WGEF interaction and corroborate our biochemical studies on the Dvl2PDZ–peptide complexes.
Furthermore, our MD studies and previously reported crystal structures of Dvl2-internal peptide complexes show no conserved mode of interaction between the internal peptides and Dvl2PDZ. In the Dvl2PDZ-N3pep crystal structure, it is observed that N3pep forms antiparallel β strand interaction with the β2 strand of the Dvl2PDZ domain (Supplementary Fig. 5a). Thus, to assess whether there is conservation of β sheet formation of the internal peptides with the β2 strand of the Dvl2PDZ domain, we calculated the secondary structural propensity of the peptide residues using their respective MD trajectories. In case of Dvl2PDZ domain and internal peptide complexes (WGEFpep, WGEFpepQ8A and N3pep peptides), it was observed that these ligands did not show consistency in forming β sheet with the β2 strand of the Dvl2PDZ domain, as the ligand may not essentially adopt β strand conformation. Perhaps this loss of secondary structural conformation might aid the flexibility of the peptide and hence, enhance its interaction with Dvl2PDZ (Supplementary Fig. 5b–d).
Dvl2PDZ exhibits ligand-dependent conformational changes
Earlier studies have shown that certain PDZ domains like ErbinPDZ and SyntrophinPDZ exhibit limited flexibility during peptide interaction. Perhaps that is why ErbinPDZ specifically accommodates C-terminal peptides with higher sequence specificity. However, SynrophinPDZ can interact with both the C-terminal as well as the internal peptides, provided the peptide undergoes a conformational change to form a β finger, in order to be incorporated within the peptide binding loop, as seen in the structure of nNOS–SyntrophinPDZ complex42,43. Another PDZ domain-containing protein, Par-6, displays allostery-mediated binding for C-terminal peptides, which exhibits an increase in affinity for the ligand by 10-folds upon binding of Cdc42 to its adjacent Cdc42/Rac interactive binding (CRIB) domain44. Par-6PDZ domain binds to the internal peptide of Pals1, as well, through a conformational change in the PDZ domain, which is independent of allosteric alterations37. Thus, it is clear from previous studies that PDZ domains exhibit huge conformational dynamics to accommodate their binding motifs. Earlier efforts have tried to characterize these dynamics using MD simulations42. It is worth mentioning that the dynamical features of PDZ domains presented from different MD studies extensively vary45. This could be due to variations in the type of PDZ structures (apo, or bound with C-terminal and/or internal peptide) employed in the MD simulations and the simulation time45,46. Since we have elucidated the apo structure of human Dvl2PDZ (PDB ID: 8WWR), we looked into the dynamics in its apo and different peptide-bound forms. It is important to mention that our hDvl2PDZ apo structure differs from the reported Xenopus Dvl2PDZ apo structures (PDB IDs: 3FY5 and 2F0A), with a substitution of just one amino acid (Human M323/Xenopus I310).
A comparison of available Dvl2PDZ structures shows deviations with RMSD values in the range of 1.7–2 Å. Importantly, significant conformational variations are observed in the α2 helix and β2–β3 loop, which are part of the peptide binding pocket. This conformational variation is more prominent in the structures of human Dvl2PDZ, bound with different peptides (Fig. 4a–c and Supplementary Fig. 5a). This opens up the question of whether the peptide binding pocket of Dvl2PDZ is preformed or it exhibits peptide-induced conformational fit. To identify the amino acids that are dynamically coupled, we performed spectral decomposition of the dynamic cross-coupling matrix (cij) (Fig. 4d) obtained from all the MD simulations. As explained in the “Methods” section, the statistical coupling analysis (SCA) provides two main clusters of dynamically coupled amino acids, referred to as Sector 1 and Sector 2. These sectors can be further resolved into five independent components (ICs) (Supplementary Fig. 6). It is interesting to note that Sector 1 corresponding to all the simulations, which primarily consists of residues belonging to the peptide-binding pocket, show significant variations in terms of their residue composition and location of residues in the protein structure (Fig. 4e–h). Interestingly, Sector 1 of apo Dvl2PDZ is composed of residues residing in β2 and α2 regions of the domain, whereas Sector 1 of Dvl2PDZ internal peptide (WGEFpep, WGEFpepQ8A and N3pep) complexes had residues from the β2 strand, β1–β2 and β2–β3 loops (Fig. 4e–h). These findings further validate the internal allostery of PDZ domains as elucidated by other studies47. Additionally, variations in Sector 1 composition highlight peptide-dependent conformational adaptation of Dvl2PDZ domain. In contrast to the earlier study by Munz et al., we observe that the α2 helix of the apo Dvl2PDZ structure has limited conformational flexibility rather than having the ability to explore all possible conformational space, specific to the ligand-bound forms. Perhaps, divergent observations of our studies from those published before could be due to the absence of a “true” apo structure of Dvl2PDZ. Furthermore, the shorter duration of earlier MD simulation (200 ns)42 might have exaggerated the conformational sampling of the PDZ domain. Thus, our structural and MD simulation studies suggest that Dvl2PDZ adopts peptide-dependent conformational change due to its intrinsic flexibility. In short, we can infer that Dvl2PDZ may not have a preformed binding pocket, rather it modulates the binding pocket based on the substrate available for binding. This adaptation is perhaps responsible for the peptide promiscuity of Dvl2PDZ.
a Apo structure of human Dvl2PDZ. b Superimposition of apo Dvl2PDZ and peptide ligand bound Dvl2PDZ structures colour coded as Dvl2PDZ (PDB ID: 8WWR)-grey, C1pep (PDB ID: 3CBX)-green, N1pep (PDB ID: 3CBY)-blue, N2pep (PDB ID: 3CBZ)-peach, N3pep (PDB ID: 3CC0)-pink, major conformational deviations in the α2 helix and β2-β3 loop regions of Dvl2PDZ are marked with a red circle. c Residue-wise RMSD plot between the apo and internal peptide ligand bound Dvl2PDZ structures. d Representative plot of the dynamic cross-coupling matrix (cij) used for calculating the cluster of dynamically coupled amino acids in peptide bound and apo Dvl2PDZ structures. e–h Dynamically coupled amino acids belonging to Sector 1 were shown as blue spheres on the apo and WGEFpep, WGEFpepQ8A and N3pep bound Dvl2PDZ structures, respectively.
Discussion
Despite nearly three decades of studies on PDZ domains, their modes of interaction with their binding partners have remained elusive. It is quite intriguing to learn that despite having highly conserved domain structure, PDZ domains exhibit divergent modes of interaction with their binding partners31,42,48. Dishevelled, being a signalling hub, recognizes diverse binding partners through its different domains, especially involving its PDZ domain49. Therefore, as seen in other PDZ domains, Dvl2PDZ exhibits significant promiscuity by recognizing binding partners having both C-terminal as well as internal binding peptide motifs27,39,50. Thus, it is expected that Dvl2PDZ displays divergent binding modes with its partners35. In the context of Wnt-PCP signalling, Dvl2 assembles with the activated Frizzled receptors and triggers RhoA by activating its GEF, i.e., WGEF. The role of Dvl2 in this signal transduction event is to activate WGEF by releasing it from its autoinhibited state. This step involves interaction between the PDZ domain of Dvl2 and WGEF. However, the particular region of WGEF that mediates its interaction with Dvl2PDZ was not known. Here, we have successfully identified an internal peptide motif of WGEF, lying between the N-terminal inhibitory and the DH domains, that facilitates the interaction of the protein with Dvl2 to activate the GEF. Indeed, this is one of the crucial mechanisms involved in releasing the autoinhibition of WGEF. Using MD simulations and comparative structural analysis, we show that Dvl2PDZ exhibits diverse modes of ligand recognition, which is perhaps true of the WGEFpep, as well. Compared to the other known Dvl2PDZ–internal peptide interactions, in the WGEF-Dvl2PDZ complex, only five residues at the P2, P0, P−1, P−2 and P−3 positions appear to contribute significantly in stabilizing their interactions (Fig. 1d). Furthermore, in terms of consensus sequence, the substitution of P11A at the P2 position of WGEFpep makes the peptide homologues to the class III PDZ binding peptide. However, in terms of the mode of binding, they differ significantly. Class III peptide bound to nNOSPDZ (PDB ID: 1B8Q)51 happens to be relatively shifted towards the N-terminal of the α2 helix of the Dvl2PDZ domain (Supplementary Fig. 7). Therefore, just based on the sequence of binding peptide mode of binding cannot be inferred. Additionally, from MD studies we observed that WGEFpep and N3pep peptides do not seem to adopt the stringent β strand conformation, as seen in case of the crystal structures of other PDZ-internal peptide complexes35. In short, the ligand (peptide) and the Dvl2PDZ undergo conformational adaptation for mutual recognition. A genome-wide screen for internal PDZ binding motifs suggests that the ability of PDZ domains to bind internal peptides is much more prevalent than previously recognized41. To comprehend how Dvl2PDZ binds to the identified binding motif within the structure of hWGEF, we looked at the AlphaFold predicted structure of hWGEF (Supplementary Fig. 8a). Clearly, major portion of the structure, including the helix that connects PDZ binding motif to the NID domain, is predicted with low confidence level (Supplementary Fig. 8b). Furthermore, the spatial disposition of different domains of the structure appears to be less reliable as the loops that connect these domains are predicted with low confidence level. However, to propose a hypothesis using this model, we superimposed the part of the hWGEF structure that is predicted with higher confidence on its closest structure homologue, Leukaemia-associated RhoGEF (PDB ID: 1X86). This comparison shows that in the absence of Dvl2PDZ interaction, the GTPase (RhoA) binding pocket of hWGEF is blocked by the N-terminal inhibitory and C-terminal SH3 domains (Supplementary Fig. 8c, d). Thus, the intra-protein interactions could render the GEF into its inactive state (Supplementary Fig. 8e). When the Dvl2PDZ domain engages with the “binding motif”, it could introduce conformational changes in the NID, resulting in it moving away from the GTPase binding pocket of the GEF. Thus, the hWGEF–Dvl2PDZ association could partially release the autoinhibitory state of the former (Supplementary Fig. 8f). Perhaps, some other unknown mechanisms might also disengage the SH3 domain from the DH domain, which would result in the complete activation of GEF (Supplementary Fig. 8g). Since the putative mechanism of hWGEF activation proposed here is based on the AlphaFold predicted structure, substantive GEF activation mechanism warrants further structural studies.
In light of this, studies reported here have thus provided invaluable insights on WGEF–Dvl2PDZ interaction and augmented the existing repository of PDZ-internal peptide interaction modes. Our study may also pave the way for designing inhibitors to selectively block Wnt-frizzled signalling, which is dysregulated in many pathological conditions52.
Methods
Peptides and constructs
Synthetic peptides such as WGEFpep, WGEFpep mutants and N3pep were procured from Sai Biotech. Fused constructs of hDvl2PDZ-WGEFpep were commercially synthesized from Twist Biosciences (https://www.twistbioscience.com).
Constructs, cloning and mutagenesis
Human Arhgef19 (encoding hWGEF 330–581) and Xenopus Arhgef19 (encoding xWGEF 330–856) genes were PCR amplified and cloned into a modified p3E vector (with N-terminal cleavable GST tag). Human RhoA (encoding hRhoA 2–180) was cloned in a modified pET33b vector (with N-terminal double Strep-tag). Human Dishevelled2 PDZ (hDvl2 PDZ) was a gift from Nicola Burgess-Brown (Addgene plasmid # 38876; RRID: Addgene_38876); this vector was modified by introducing a stop codon after C354 residue of Dvl2PDZ to eliminate the fused peptide, cloned at the C-termini of PDZ. This construct has an N-terminal cleavable 6xHis tag. Mutations were introduced into xWGEF (330–856) through site-directed mutagenesis method and confirmed by DNA sequencing.
Protein expression and purification
Recombinant hWGEF (330–581) and RhoA (2–180) were expressed into Escherichia coli (E. coli) strain BL21*(DE3), whereas xWGEF (330–856) was expressed into C41 (DE3) strain. Cells were grown at 37 °C in Luria Broth (LB) containing Ampicillin (100 µg/mL) followed by induction with 0.5 mM isopropyl β-d-thiogalactopyranoside (IPTG) for 16 h at 18 °C once the OD (optical density at 600 nm) of 0.6 is reached. After incubation, cells were pelleted at 4000 rpm for 20 min and stored at −80 °C. GST-tagged proteins were purified using glutathione (GSH) Sepharose-4B affinity purification followed by dialysis and GST tag cleavage using PreScission protease at 4 °C overnight. The GST tag and PreScission protease were removed by desalting, followed by a second GST affinity purification. To obtain stable and pure protein, gel filtration chromatography was performed using Hiprep 26/60 Sephacryl S-300 HR SEC column in a buffer containing 10 mM Tris–HCl pH 8, 150 mM NaCl, 2 mM DTT and 5% glycerol for xWGEF. Hiprep 16/60 Sephacryl S-200 HR SEC column with 10 mM MOPS pH 6.5, 150 mM NaCl, 2 mM DTT and 10% Glycerol buffer was used for gel filtration chromatography of hWGEF. RhoA was purified using streptactin affinity purification, after which the protein was subjected to gel filtration chromatography using Hiprep 16/60 Sephacryl S-200 HR SEC column in the buffer containing 10 mM Tris–HCl pH 8, 150 mM NaCl, 2mM EDTA, 2 mM DTT and 5% glycerol. 6xHis tagged hDvl2PDZ and fused hDvl2PDZ-WGEFpep constructs were expressed in Rosetta (DE3) cells and purified using Ni2+-nitrilotriacetate (Ni2+-NTA). 6xHis tag is further cleaved by using TEV protease during overnight dialysis. TEV protease and His tag are eliminated through desalting and 2nd Ni2+-NTA purification. Finally, pure protein is obtained after gel filtration using Hiprep 16/60 Sephacryl S-200 HR SEC column in buffer containing 10 mM Tris–HCl pH 8, 150 mM NaCl, 2 mM DTT and 5% glycerol for hDvl2PDZ and 10 mM Tris–HCl pH 8, 350 mM NaCl, 2 mM DTT and 5% glycerol for hDvl2PDZ-WGEFpep. Mutations were introduced into xWGEF (330–856) through site-directed mutagenesis; expression and purification were carried out as described above for the GST-tagged proteins. All proteins were concentrated and stored at −80 °C for further studies.
Protein crystallization, data collection and structure determination
Crystals of Human Dvl2PDZ (hDvl2PDZ) were grown with sitting drop vapour diffusion method at 293 K by mixing 1:1 ratio of hDvl2PDZ (10 mg/mL) with 6 M excess of WGEFpep and reservoir buffer containing 0.1 M tri-sodium citrate (pH 5.6), 20% (v/v) Isopropanol, 20% (w/v) PEG4000. Crystals of Human Dvl2PDZ fused with WGEFpep (hDvl2PDZ-WGEFpep) were grown with sitting drop vapour diffusion method at 293 K by mixing 1:1 ratio of protein (10 mg/mL) and reservoir buffer containing 0.1 M Sodium acetate (pH 4.5) and 3 M Sodium chloride. The crystallization plate was set up using a Mosquito® crystallization robot (TTP Labtech, Royston UK). Crystals were frozen in cryoprotectants containing crystallization conditions supplemented with 28% glycerol. X-ray diffraction data was collected at 100 K on microfocus beamline ID23-2 of the European Synchrotron Radiation Facility (ESRF), Grenoble, France53. Crystals of the fused hDvl2PDZ-WGEFpep protein were diffracted at Indus-2 synchrotron on BL-21 PX beamline at RRCAT, Indore. All the data sets were integrated with XDS54 and scaled using AIMLESS55,56, implemented on CCP4 software57. The structure was solved by molecular replacement with PHASER58. Human Dvl2-PDZ structure (PDB entry: 2REY) served as a search model. The structure was further iteratively built using COOT59 and refined using PHENIX60,61. The structure validation was performed using Molprobidity62. Coordinates and structure factors for hDvl2PDZ and WGEFpep fused hDvl2PDZ are deposited in the Protein Data Bank under accession code: PDB IDs 8WWR and 8YR7, respectively.
In vitro guanine nucleotide exchange assay
Guanine nucleotide exchange assays were carried out using RhoA loaded with fluorescently labelled Mant-GDP63,64. Briefly, 150 μM of RhoA was incubated with 5 Molar excess of fluorescent label, i.e., Mant-GDP in a buffer containing 10 mM Tris pH 8, 150 mM NaCl, 2 mM DTT, 5 mM EDTA, 0.5 mM MgCl2 and 5% Glycerol. The reaction was incubated on ice for 30 min, followed by the addition of 10 mM MgCl2 to stop the reaction. Excess Mant-GDP was removed through buffer exchange and Mant-GDP loaded RhoA was concentrated and stored at −80 °C. For assays, 2 μM of Mant-GDP loaded RhoA was incubated with 5 μM of xWGEF in the presence or absence of hDvl2PDZ (0 μM, 25 μM and 50 μM) for 30 min at 25 °C. To observe effect of the synthetic peptides WGEFpep (GSTFSLWQDIP) and WGEFpepD9A (GSTFSLWQAIP) on Dvl2PDZ–WGEF interaction, these peptides were added to a final concentration of 200 μM in the respective reactions. Once the fluorescence was stable, excess GTP (10x of labelled GTPase) was added to start the exchange reaction. All the GEF assays were performed in a buffer containing 10 mM Tris, pH 8, 150 mM NaCl, 2 mM DTT, 10 mM MgCl2 and 5% Glycerol. A decrease in fluorescence was recorded using a BioTek Cytation5 plate reader with excitation and emission of 360 and 440 nm, respectively. Fluorescence was normalized and decrease was fitted using a single exponential decay mode in GraphPad Prism. All the assays were performed in triplicates.
Microscale thermophoresis (MST)
MST experiments were performed using Monolith NT.115 instrument from Nanotemper technologies. Dvl2PDZ domain was fluorescently labelled as per the instructions, using Monolith Redmaleimide 2nd generation-cysteine reactive label. The experimental condition consists of 10 mM Tris–HCl pH8, 150 mM NaCl, 2 mM DTT, 5% Glycerol and 0.05% Tween20. For Dvl2PDZ-peptide affinity determination, synthetic WGEF peptides were serially diluted (1:2) 16 times, starting from an approximate concentration of 3.4 mM. 50 nM of fluorescently labelled Dvl2PDZ domain was added to the serially diluted peptides, followed by incubation at 25 °C for 30 minutes. Thermophoresis signals were recorded at 20% of excitation power and 40% MST at 25 °C. Data was fitted using the Kd fit model in the MO. Affinity Analysis v2.3 software65. Like peptides, hWGEF (330–581) protein was serially diluted to obtain a protein concentration range of 138–0.0042 µM. Experimental conditions were the same as mentioned above for peptides. All MST experiments were done in triplicates.
Molecular dynamics (MD) simulations of hDvl2PDZ and interacting peptides
MD simulations were performed by using the Desmond module from the Schrödinger suite66. The crystal structure of apo human Dvl2PDZ (PDB code: 8WWR) was used as a template for MODELLER-based homology modelling in order to build the missing loops67. WGEFpep, WGEFpepQ8A mutant peptide and N3pep peptides were modelled and docked on Dvl2PDZ by using the previously available internal (N3pep) peptide structure (PDB code: 3CC0). These peptides, along with the modelled Dvl2PDZ structure, were further used for 1 µs MD simulation. Apo form of Dvl2PDZ was also simulated by adopting a similar methodology. All the structures were placed in a simulation box of 10 Å and further solvated using TIP3P water molecules along with Na+ ions to neutralize the system68. Next, temperature equilibration was done at a constant temperature of 300 K using the Nosé–Hoover thermostat, and the pressure was equilibrated at 1.01 bar under NPT ensemble using Martyna-Tobias-Klein barostat69,70,71,72. Followed by temperature and pressure equilibration, MD runs were performed using OPLS_2005 force73,74. MD runs were performed in triplicates. Other parameters used for simulations are mentioned in Supplementary Table 2. RMSD plots for Dvl2PDZ and peptides are given in Supplementary Fig. 9.
Dynamical coupling analysis
The dynamical cross-correlation matrix (DCCM), dCij75, was calculated as
where \({r}_{i}\) and \({r}_{j}\) are the position vectors of the ith and jth Cα atoms, respectively of Dvl2PDZ, at time t. The R implementation of the Bio3D programme (version 2.4-1)76 was used for the DCCM calculations. The dynamical sectors were obtained from the spectral decomposition algorithm77 using the methodology (statistical coupling analysis) reported earlier64,78. Trajectories from all the triplicate MD runs were merged for dynamical coupling analysis.
Statistics and reproducibility
Guanine nucleotide exchange assays were performed in triplicates. Fluorescence decrease was fitted using a single exponential decay mode in GraphPad Prism. All results are reported with standard deviation (±SD). Statistical significance was calculated using ordinary One-way ANOVA with multiple comparisons. * Represents adjust P value with statistical significance of ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P ≤ 0.01. MST assays were performed in triplicates. Data was fitted using the Kd fit model in the MO. Affinity Analysis v2.3 software. All results are reported with standard deviation (±SD). MD simulation runs for apo and peptide docked Dvl2PDZ were performed in triplicates. Plots reported from MD simulation studies are represented with standard deviation (±SD).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The atomic model of apo human Dvl2 PDZ is available in the Protein Data Bank (PDB) under the accession code 8WWR. The atomic model of human Dvl2 PDZ fused with WGEFpep (no electron density for the peptide) is available in the Protein Data Bank (PDB) under the accession code 8YR7. Initial coordinates, simulation input files and final coordinates of the MD simulation runs are available at https://zenodo.org/records/1068373179. The source data for graphs in the manuscript can be found in Supplementary Data 1.
References
Logan, C. Y. & Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004).
Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 7, 3 (2022).
Chae, W. J. & Bothwell, A. L. M. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 39, 830–847 (2018).
Komiya, Y. & Habas, R. Wnt signal transduction pathways. Organogenesis 4, 68–75 (2008).
Groenewald, W., Lund, A. H. & Gay, D. M. The role of WNT pathway mutations in cancer development and an overview of therapeutic options. Cells 12, 990 (2023).
Patel, S., Alam, A., Pant, R. & Chattopadhyay, S. Wnt signaling and its significance within the tumor microenvironment: novel therapeutic insights. Front. Immunol. 10, 486317 (2019).
Schlessinger, K., Hall, A. & Tolwinski, N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 23, 265–277 (2009).
Kjøller, L. & Hall, A. Signaling to Rho GTPases. Exp. Cell Res. 253, 166–179 (1999).
Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Omble, A. & Kulkarni, K. GPCRs that Rhoar the Guanine nucleotide exchange factors. Small GTPases 13, 84–99 (2022).
Schiller, M. R. Coupling receptor tyrosine kinases to Rho GTPases-GEFs what’s the link. Cell. Signal. 18, 1834–1843 (2006).
Ridley, A. Rho GTPases: Integrating integrin signaling. J. Cell Biol. 150, F107–F109 (2000).
Wang, Y. et al. WGEF is a novel RhoGEF expressed in intestine, liver, heart, and kidney. Biochem. Biophys. Res. Commun. 324, 1053–1058 (2004).
Kim, K., Lee, S. A. & Park, D. Emerging roles of ephexins in physiology and disease. Cells 8, 87 (2019).
Hardy, K. M. et al. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev. Biol. 320, 391–401 (2008).
Tada, M., Concha, M. L. & Heisenberg, C. P. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin. Cell Dev. Biol. 13, 251–260 (2002).
Tanegashima, K., Zhao, H. & Dawid, I. B. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 27, 606–617 (2008).
Hu, D. J. K., Yun, J., Elstrott, J. & Jasper, H. Non-canonical Wnt signaling promotes directed migration of intestinal stem cells to sites of injury. Nat. Commun. 12, 7150 (2021).
Sahin, M. et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46, 191–204 (2005).
Yohe, M. E. et al. Auto-inhibition of the Dbl family protein Tim by an N-terminal helical motif. J. Biol. Chem. 282, 13813–13823 (2007).
Yohe, M. E., Rossman, K. & Sondek, J. Role of the C-terminal SH3 domain and N-terminal tyrosine phosphorylation in regulation of Tim and related Dbl-family proteins. Biochemistry 47, 6827–6839 (2008).
Kim, K. et al. Intermolecular steric inhibition of Ephexin4 is relieved by Elmo1. Sci. Rep. 7, 4404 (2017).
Kim, K. et al. The intermolecular interaction of Ephexin4 leads to autoinhibition by impeding binding of RhoG. Cells 7, 211 (2018).
Gao, C. & Chen, Y. G. Dishevelled: the hub of Wnt signaling. Cell. Signal. 22, 717–727 (2010).
Sharma, M., Castro-Piedras, I., Simmons, G. E. & Pruitt, K. Dishevelled: a masterful conductor of complex Wnt signals. Cell. Signal. 47, 52–64 (2018).
Wallingford, J. B. & Habas, R. The developmental biology of dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132, 4421–4436 (2005).
Wong, H. C. et al. Direct binding of the PDZ domain of dishevelled to a conserved internal sequence in the C-terminal region of frizzled. Mol. Cell 12, 1251–1260 (2003).
Paclíková, P., Bernatík, O., Radaszkiewicz, T. W. & Bryja, V. The N-terminal part of the dishevelled DEP domain is required for Wnt/β-Catenin signaling in mammalian cells. Mol. Cell. Biol. 37, e00145–17 (2017).
Schwarz-Romond, T. et al. The DIX domain of dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484–492 (2007).
Nielsen, C. P., Jernigan, K. K., Diggins, N. L., Webb, D. J. & MacGurn, J. A. USP9X deubiquitylates DVL2 to regulate WNT pathway specification. Cell Rep. 28, 1074–1089 (2019).
Lee, H. J. & Zheng, J. J. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun. Signal. 8, 1–18 (2010).
Songyang, Z. et al. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275, 73–77 (1997).
Ali, M. et al. Integrated analysis of Shank1 PDZ interactions with C-terminal and internal binding motifs. Curr. Res. Struct. Biol. 3, 41–50 (2021).
Zhu, Y. et al. Deciphering the unexpected binding capacity of the third PDZ domain of whirlin to various cochlear hair cell partners. J. Mol. Biol. 432, 5920–5937 (2020).
Zhang, Y. et al. Inhibition of Wnt signaling by dishevelled PDZ peptides. Nat. Chem. Biol. 5, 217–219 (2009).
Aasland, R. et al. Normalization of nomenclature for peptide motifs as ligands of modular protein domains. FEBS Lett. 513, 141–144 (2002).
Penkert, R. R., DiVittorio, H. M. & Prehoda, K. E. Internal recognition through PDZ domain plasticity in the Par-6–Pals1 complex. Nat. Struct. Mol. Biol. 11, 1122–1127 (2004).
Skelton, N. J. et al. Origins of PDZ domain ligand specificity: structure determination and mutagenesis of the erbin PDZ domain. J. Biol. Chem. 278, 7645–7654 (2003).
Lee, H. J., Shi, D. L. & Zheng, J. J. Conformational change of dishevelled plays a key regulatory role in the wnt signaling pathways. Elife 4, e08142 (2015).
Ivarsson, Y. Plasticity of PDZ domains in ligand recognition and signaling. FEBS Lett. 586, 2638–2647 (2012).
Mu, Y., Cai, P., Hu, S., Ma, S. & Gao, Y. Characterization of diverse internal binding specificities of PDZ domains by yeast two-hybrid screening of a special peptide library. PLoS ONE 9, e88286 (2014).
Münz, M., Hein, J. & Biggin, P. C. The role of flexibility and conformational selection in the binding promiscuity of PDZ domains. PLoS Comput. Biol. 8, e1002749 (2012).
Hillier, B. J., Christopherson, K. S., Prehoda, K. E., Bredt, D. S. & Lim, W. A. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 284, 812–815 (1999).
Whitney, D. S., Peterson, F. C. & Volkman, B. F. A conformational switch in the CRIB-PDZ module of Par-6. Structure 19, 1711–1722 (2011).
Lu, C., Knecht, V. & Stock, G. Long-range conformational response of a PDZ domain to ligand binding and release: a molecular dynamics study. J. Chem. Theory Comput. 12, 870–878 (2016).
Amacher, J. F., Brooks, L., Hampton, T. H. & Madden, D. R. Specificity in PDZ-peptide interaction networks: computational analysis and review. J. Struct. Biol. X. 4, 100022 (2020).
Stevens, A. O. & He, Y. Allosterism in the PDZ family. Int. J. Mol. Sci. 23, 1454 (2022).
Harris, B. Z. & Lim, W. A. Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci. 114, 3219–3231 (2001).
Wharton, K. A. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253, 1–17 (2003).
Tonikian, R. et al. A specificity map for the PDZ domain family. PLoS Biol. 6, e239 (2008).
Tochio, H., Zhang, Q., Mandal, P., Li, M. & Zhang, M. Solution structure of the extended neuronal nitric oxide synthase PDZ domain complexed with an associated peptide. Nat. Struct. Biol. 6, 417–421 (1999).
Katoh, M. WNT/PCP signaling pathway and human cancer. Oncol. Rep. 14, 1583–1588 (2005).
Flot, D. et al. The ID23-2 structural biology microfocus beamline at the ESRF. J. Synchrotron Radiat. 17, 107–118 (2010).
Kabsch, W. et al. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. Sect. D Biol. Crystallogr. 67, 282–292 (2011).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. Sect. D Biol. Crystallogr. 69, 1204–1214 (2013).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. Sect. D Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 213–221 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. Sect. D Struct. Biol. 75, 861–877 (2019).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Kanie, T. & Jackson, P. Guanine nucleotide exchange assay using fluorescent MANT-GDP. Bio-Protocol 8, e2795–e2795 (2018).
Roy, D., Sengupta, D. & Kulkarni, K. Substrate induced dynamical remodeling of the binding pocket generates GTPase specificity in DOCK family of guanine nucleotide exchange factors. Biochem. Biophys. Res. Commun. 631, 32–40 (2022).
Asmari, M., Ratih, R., Alhazmi, H. A. & El Deeb, S. Thermophoresis for characterizing biomolecular interaction. Methods 146, 107–119 (2018).
Bowers, K. J. et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In SC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing. (ed. Horner-Miller, B.) 43 (IEEE, 2006).
Šali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Evans, D. J. & Holian, B. L. The Nose–Hoover thermostat. J. Chem. Phys. 83, 4069–4074 (1985).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Martyna, G. J., Tobias, D. J. & Klein, M. L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).
Martyna, G. J., Tuckerman, M. E., Tobias, D. J. & Klein, M. L. Explicit reversible integrators for extended systems dynamics. Mol. Phys. 87, 1117–1157 (1996).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Shivakumar, D. et al. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the opls force field. J. Chem. Theory Comput. 6, 1509–1519 (2010).
Karplus, M. & Ichiye, T. Comment on a “Fluctuation and cross-correlation analysis of protein motions observed in nanosecond molecular dynamics simulations”. J. Mol. Biol. 263, 120–122 (1995).
Grant, B. J., Rodrigues, A. P. C., Elsawy, K. M., Mccammon, J. A. & Caves, L. S. D. Bio3d: an R package for the comparative analysis of protein structures. Bioinformatics 22, 2695–2696 (2006).
Rivoire, O., Reynolds, K. A. & Ranganathan, R. Evolution-based functional decomposition of proteins. PLoS Comput. Biol. 12, e1004817 (2016).
Singh, S., Thulasiram, H. V., Sengupta, D. & Kulkarni, K. Dynamic coupling analysis on plant sesquiterpene synthases provides leads for the identification of product specificity determinants. Biochem. Biophys. Res. Commun. 536, 107–114 (2021).
Omble, A., Mahajan, S., Bhoite, A. & Kulkarni, K. Dishevelled2 activates WGEF via its interaction with a unique internal peptide motif of the GEF [Dataset]. Zenodo https://zenodo.org/records/10683731 (2024).
Acknowledgements
K.K. would like to acknowledge funding from the Department of Biotechnology, India (BT/PR12502/BRB/10/ 1387/2015). We thank Prof. Dawid Igor for generously sharing various constructs of Dvl, Frizzled and WGEF. We acknowledge the European Synchrotron Radiation Facility (ESRF), ID23-2 beamline, for the provision of synchrotron radiation facilities and we would like to thank Dr. Christoph Mueller-Dieckmann for assistance and support in using the beamline. We acknowledge BL-21 PX beamline scientists Dr. Ravindra D. Makde, Dr. Ashwani Kumar and Dr. Biplab Ghosh at RRCAT, Indore, India, for helping with data collection. The authors acknowledge the Central Instrumentation Facility (CIF), Savitribai Phule Pune University (SPPU) for the MST facility. The authors acknowledge Dr. Durba Sengupta for their inputs on MD simulations. A.O. acknowledges the Council of Scientific & Industrial Research (CSIR), India for fellowship.
Author information
Authors and Affiliations
Contributions
A.O. designed and performed all the experiments. S.M. helped with the crystallization studies. A.B. screened the initial constructs of WGEF for expression and solubility. K.K. conceptualized the problem, supervised overall work, analysed the data and wrote the paper with inputs from A.O.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Huijuan Guo, Tobias Goris, and David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omble, A., Mahajan, S., Bhoite, A. et al. Dishevelled2 activates WGEF via its interaction with a unique internal peptide motif of the GEF. Commun Biol 7, 543 (2024). https://doi.org/10.1038/s42003-024-06194-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06194-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.