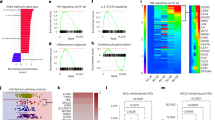
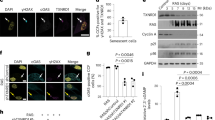
Abstract
Aging is characterized by an increased vulnerability to infection and the development of inflammatory diseases, such as atherosclerosis, frailty, cancer and neurodegeneration. Here, we find that aging is associated with the loss of diurnally rhythmic innate immune responses, including monocyte trafficking from bone marrow to blood, response to lipopolysaccharide and phagocytosis. This decline in homeostatic immune responses was associated with a striking disappearance of circadian gene transcription in aged compared to young tissue macrophages. Chromatin accessibility was significantly greater in young macrophages than in aged macrophages; however, this difference did not explain the loss of rhythmic gene transcription in aged macrophages. Rather, diurnal expression of Kruppel-like factor 4 (Klf4), a transcription factor (TF) well established in regulating cell differentiation and reprogramming, was selectively diminished in aged macrophages. Ablation of Klf4 expression abolished diurnal rhythms in phagocytic activity, recapitulating the effect of aging on macrophage phagocytosis. Examination of individuals harboring genetic variants of KLF4 revealed an association with age-dependent susceptibility to death caused by bacterial infection. Our results indicate that loss of rhythmic Klf4 expression in aged macrophages is associated with disruption of circadian innate immune homeostasis, a mechanism that may underlie age-associated loss of protective immune responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Transcriptomics data are available under accession number GSE128830 and at Token (ybqjocoontgtluf); ATAC-seq data are available at https://purl.stanford.edu/rc797bt9574. The dataset used for the analyses in the UK BioBank have not been deposited in a public repository but are available after approval of a reasonable application at https://www.ukbiobank.ac.ukl. Source data are provided with this paper.
References
Bhadra, U., Thakkar, N., Das, P. & Pal Bhadra, M. Evolution of circadian rhythms: from bacteria to human. Sleep Med. 35, 49–61 (2017).
Herzog, E. D. & Tosini, G. The mammalian circadian clock shop. Semin. Cell Dev. Biol. 12, 295–303 (2001).
Storch, K. F. et al. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 (2002).
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014).
Fonken, L. K. et al. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav. Immun. 45, 171–179 (2015).
He, W. et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity 49, 1175–1190 (2018).
Keller, M. et al. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl Acad. Sci. USA 106, 21407–21412 (2009).
Nguyen, K. D. et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 341, 1483–1488 (2013).
Geiger, S. S., Curtis, A. M., O’Neill, L. A. J. & Siegel, R. M. Daily variation in macrophage phagocytosis is clock-independent and dispensable for cytokine production. Immunology 157, 122–136 (2019).
Kitchen, G. B. et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl Acad. Sci. USA 117, 1543–1551 (2020).
Oliva-Ramírez, J., Moreno-Altamirano, M. M. B., Pineda-Olvera, B., Cauich-Sánchez, P. & Javier Sánchez-García, F. Crosstalk between circadian rhythmicity, mitochondrial dynamics and macrophage bactericidal activity. Immunology 143, 490–497 (2014).
Curtis, A. M. et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl Acad. Sci. USA 11, 7231–7236 (2015).
Hayashi, M., Shimba, S. & Tezuka, M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 30, 621–626 (2007).
Knyszynski, A. & Fischer, H. Circadian fluctuations in the activity of phagocytic cells in blood, spleen, and peritoneal cavity of mice as measured by zymosan-induced chemiluminescence. J. Immunol. 127, 2508–2511 (1981).
Leone, M. J., Marpegan, L., Duhart, J. M. & Golombek, D. A. Role of proinflammatory cytokines on lipopolysaccharide-induced phase shifts in locomotor activity circadian rhythm. Chronobiol. Int. 29, 715–723 (2012).
Rahman, S. A. et al. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav. Immun. 47, 4–13 (2015).
Huo, M. et al. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 31, 1097–1106 (2017).
Scheiermann, C., Kunisaki, Y. & Frenette, P. S. Circadian control of the immune system. Nat. Rev. Immunol. 13, 190–198 (2013).
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Liu, Q. et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat. Immunol. 20, 1023–1034 (2019).
Deng, W. et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 24, 366–378 (2018).
Scheiermann, C. et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301 (2012).
Hughes, M. E., Hogenesch, J. B. & Kornacker, K. JTK-CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythms 25, 372–380 (2010).
Palmieri, M. et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 (2011).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550–550 (2014).
Moskowitz, D. M. et al. Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol. 2, eaag0192 (2017).
Ucar, D. et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 214, 3123–3144 (2017).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. ChromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008).
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007).
Ipseiz, N. et al. Effective in vivo gene modification in mouse tissue-resident peritoneal macrophages by intraperitoneal delivery of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 16, 21–31 (2020).
Liu, H. et al. Irf6 directly regulates Klf17 in zebrafish periderm and Klf4 in murine oral epithelium, and dominant-negative KLF4 variants are present in patients with cleft lip and palate. Hum. Mol. Genet. 25, 766–776 (2016).
Stratopoulos, A. et al. Genomic variants in members of the Krüppel-like factor gene family are associated with disease severity and hydroxyurea treatment efficacy in β-hemoglobinopathies patients. Pharmacogenomics 20, 791–801 (2019).
Stremitzer, S. et al. Genetic variants associated with colorectal brain metastases susceptibility and survival. Pharmacogenomics J. 17, 29–35 (2017).
Soufi, A. et al. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 161, 555–568 (2015).
Di Giammartino, D. C. et al. KLF4 is involved in the organization and regulation of pluripotency-associated three-dimensional enhancer networks. Nat. Cell Biol. 21, 1179–117 (2019).
McConnell, B. B., Ghaleb, A. M., Nandan, M. O. & Yang, V. W. The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. BioEssays 29, 549–557 (2007).
Alder, J. K. et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J. Immunol. 180, 5645–5652 (2008).
Kapoor, N. et al. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J. Immunol. 194, 6011–6023 (2015).
Liao, X. et al. Krüppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 (2011).
Dykstra, B. & de Haan, G. Hematopoietic stem cell aging and self-renewal. Cell Tissue Res. 331, 91–101 (2008).
Dykstra, B., Olthof, S., Schreuder, J., Ritsema, M. & de Haan, G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 208, 2691–2703 (2011).
Minhas, P. S. et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Misharin, A. V., Saber, R. & Perlman, H. Eosinophil contamination of thioglycollate-elicited peritoneal macrophage cultures skews the functional readouts of in vitro assays. J. Leukoc. Biol. 92, 325–331 (2012).
Zhang, X., Goncalves, R. & Mosser, D. M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 14, Unit 14.1 (2008).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106–R106 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Thaben, P. F. & Westermark, P. O. Detecting rhythms in time series with RAIN. J. Biol. Rhythms 29, 391–400 (2014).
Hutchison, A. L., Allada, R. & Dinner, A. R. Bootstrapping and empirical Bayes methods improve rhythm detection in sparsely sampled data. J. Biol. Rhythms 33, 339–349 (2018).
Wu, G., Anafi, R. C., Hughes, M. E., Kornacker, K. & Hogenesch, J. B. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics 32, 3351–3353 (2016).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Acknowledgements
This work was supported by RO1AG048232 (K.I.A.), RF1AG058047 (K.I.A.), the American Heart Association 19PABH134580007 (K.I.A.), RO1NS100180 (K.I.A.), 1P30 AG066515 (K.I.A.), The Zhang-Jiang Research Fund, T32 Neuroscience Institute (C.T.), NSF GRFP (C.T.), DP2AG067492 (C.A.T.), the Edward Mallinckrodt, Jr. Foundation (C.A.T.), UPenn Institute for Immunology (C.A.T.), UPenn Diabetes Research Center P30-DK-019525 (C.A.T.), Pew Biomedical Scholarship (C.A.T.), Fritz Thyssen Foundation (C.A.T.) and the UPenn Institute on Aging (C.A.T.), Marie Skłodowska-Curie Grant 888494 (E.B.), Stanford School of Medicine Dean’s Postdoctoral Fellowship (E.B.), Medical Scientist Training Program T32 GM07170 (L.L.) and Training Grant in Computational Biology 5-T32-HG-000046-21 (L.L.). We thank L. de Lecea for support in housing mice, the Stanford Shared FACS facility for flow cytometry analysis on LSR instruments (S10RR027431-01) and SCGPM for sequencing on a HiSeq 2000. The ATAC-seq sequencing data were generated on an Illumina HiSeq 4000 that was purchased with funds from NIH S10OD018220 for the Stanford Functional Genomics Facility. Schematic illustrations were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
E.B. designed, performed and interpreted the experiments and wrote the manuscript. C.T. conceived the study, performed and analyzed RNA-seq experiments and wrote the manuscript. L.L., Z.S., K.M.S. and V.M. performed computational analyses. C.A.I. assisted with FACS and qRT–PCR experiments. B.C. and H.C.H. assisted with circadian experiments. K.I.A. and C.A.T. conceived the study, supervised the participants, interpreted the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Nicolas Cermakian, Anne Curtis, Lora Hooper, Luz Navarro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. L.A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Gating strategy for monocytes and macrophages in bone marrow, blood and spleen.
Mononuclear cells from bone marrow (a) blood (b) and spleen (c) were gated for forward and side-scatter (FSC/SSC), doublets, and live/dead prior to identification of bone marrow (CD45 + CD11b + Ly6G-Ly6C + ), blood (CD45 + CD115 + Ly6G-) and splenic (CD45 + CD11b + Ly6G-) monocytes and macrophages.
Extended Data Fig. 2 Macrophage enrichment validation and RNA-seq quality control and analysis.
a. Gating strategy for measurement of macrophage enrichment of samples at ZT0 and ZT12. b. Percent of live Cd11b+ macrophages in young vs aged at ZT0 and ZT12 (n = 3, 2-way ANOVA age factor p = 0.0065). c. Expression levels of macrophage transcripts, Itgam and Emr1 versus markers of dendritic cells (Itgax), eosinophils (Siglec F), T cells (Cd3d, Cd3e, Cd3g), and B cells (Cd19). Data are mean ± s.e.m, two-sided Mann-Whitney U test. n = 21 mice in each age group and n = 3 in each time group. d-k. Pooled values (d, f, h, j) and time-dependent presentations (e, g, i, k) of sample total cell counts (d-e), input RNA quantity (f-g), RIN scores (h-i) and number of mapped reads (j-k) of circadian RNA-seq analysis of young and aged peritoneal macrophages. (d, f, h, j) Data are mean ± s.e.m, two-sided Mann-Whitney U test. n = 21 mice in each age group and n = 3 in each time group. (e, g, i, k) Lines of best fit were determined using loess, and 95% confidence intervals are shown.
Extended Data Fig. 3 Algorithm comparison and amplitude assessment for rhythmic transcripts.
a. Venn diagrams of unique and shared rhythmically expressed transcripts in young vs. aged macrophages, compared across different algorithms (JTK_CYCLE, RAIN, BooteJTK, and MetaCycle) and q-values. b. JTK_CYCLE amplitudes of genes in peritoneal macrophages from young and aged mice. Genes are binned by their rhythmicity in neither, both, or individual groups. n = 21 mice in each age group and n = 3 in each time group. Boxes extend from the 25th-75th percentiles, whiskers extend to 1.5 times the IQR, and the center line is the median.
Extended Data Fig. 4 Loss of circadian rhythmicity of phagocytosis gene expression in aged macrophages.
a. Heatmap of normalized CLEAR network gene expression values of all time points for each age group shows a decrease in overall gene expression in aged as compared to young peritoneal macrophages. b. Circadian RNA-seq expression patterns of phagocytosis-related genes reveal loss of rhythmicity in aged peritoneal as compared to young macrophages. Data are mean ± s.e.m, n = 21 mice in each age group and n = 3 in each time group. Indicated p-values were calculated by JTK_CYCLE on normalized expression values.
Extended Data Fig. 5 The core circadian clock genes remain rhythmic in aged peritoneal macrophages.
a-j, Individual representations of normalized circadian gene expression values measured by RNA-seq of the positive arm (a-b), negative arm (c-g) and supporting genes (h-j) of the core clock machinery. (k-l) qPCR validation of Bmal1 and Per2 expression in young and aged peritoneal macrophages. Data are mean ± s.e.m, n = 21 mice in each age group and n = 3 in each time group.
Extended Data Fig. 6 Chromatin accessibility of young and aged macrophages is not rhythmic.
a. Schematic of investigated possible explanations for differentially rhythmic gene expression by chromatin accessibility. b. Chromatin accessibility (as normalized and log2-transformed values) of promoter regions of differentially rhythmic genes between young and aged peritoneal macrophages. Note that rhythmically expressed genes have higher chromatin accessibility compared to an equal number of randomly selected control genes. n = 15-16 mice in each age group and n = 2-3 mice per 4 h time interval. Boxes extend from the 25th-75th percentiles, whiskers extend to 1.5 times the IQR, and the center line is the median. c. Venn diagrams of the numbers of differentially rhythmic genes between the two age groups (RNA-seq) and the numbers of differentially accessible promoter regions assessed by ATAC-seq. d, Scatterplots of amplitude and q-value for open chromatin peaks as assessed by JTK_CYCLE. No element shows q < 0.2 (indicated by dotted line). e, f, Distribution of unadjusted p-values for oscillations of open chromatin peaks (e) and transcripts (f) in young macrophages, zoomed-in to p < 0.1, JTK_CYCLE.
Extended Data Fig. 7 Two distinct DNA binding motifs of KLF4.
a, b. Circadian expression levels measured by RNA-seq of KLF family members Klf9 and Klf13. Data are mean ± s.e.m, n = 21 mice in each age group and n = 3 in each time group. p-values determined by JTK_CYCLE on normalized expression values. c, d. Depiction of the two known KLF4 binding motifs, MA0039.1 and MA0039.2. q-values from de novo motif discovery on a KLF4 ChIP-seq experiment from ENCODE (https://factorbook.org/experiment/ENCSR265WJC/motif). e. chromVAR30 deviations within all 500 bp peaks indicating KLF4 binding by estimating accessibility within peaks sharing the MA0039.2 motif or annotation. p = 0.935, two-sided Mann-Whitney U test. f. chromVAR30 deviations within peaks associated with differentially rhythmic genes indicating KLF4 binding by estimating accessibility within peaks sharing the MA0039.2 motif or annotation. p = 0.654, two-sided Mann-Whitney U test. (e, f) n = 15-16 mice in each age group and n = 2-3 mice per 4 h time interval. Boxes extend from the 25th-75th percentiles, whiskers extend to 1.5 times the IQR, and the center line is the median.
Extended Data Fig. 8 Klf4 is controlled by Bmal1 and drives circadian rhythmicity of phagocytosis.
a-d. Circadian RT-PCR expression patterns of Klf4 (a-b), and the phagocytosis-related genes Gba (c) and Rab3d (d) reveal loss of rhythmicity of phagocytosis genes in Klf4-shRNA lentiviral injected young mice as compared to scrambled vector controls. Data are mean ± s.e.m, n = 18 mice in each treatment group and n = 3 in each time group. Indicated p-values were calculated by JTK_CYCLE. e-f. RT-PCR expression of Bmal1 (e) and Circadian RT-PCR expression patterns of Klf4 in Bmal1-shRNA lentiviral injected young mice as compared to scrambled vector controls. Data are mean ± s.e.m, n = 18 mice in each treatment group and n = 3 in each time group. Indicated p-values were calculated by JTK_CYCLE. g. Phagocytosis of fluorescent E. coli particles by Bmal1-shRNA lentiviral and scrambled vector injected young mice. Data are mean ± s.e.m, n = 18 mice in each treatment group and n = 3 in each time group. Indicated p-values were calculated by JTK_CYCLE. h. Crystal structure of the zinc-finger domain of KLF4 in complex with DNA and rs2236599 synonymous mutation site predicted by SWISS-MODEL57.
Supplementary information
Supplementary Information
Supplementary Table 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Blacher, E., Tsai, C., Litichevskiy, L. et al. Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol 23, 229–236 (2022). https://doi.org/10.1038/s41590-021-01083-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-01083-0
This article is cited by
-
KLF5 regulates actin remodeling to enhance the metastasis of nasopharyngeal carcinoma
Oncogene (2024)
-
Association between circadian physical activity trajectories and incident type 2 diabetes in the UK Biobank
Scientific Reports (2024)
-
Myeloid deficiency of the intrinsic clock protein BMAL1 accelerates cognitive aging by disrupting microglial synaptic pruning
Journal of Neuroinflammation (2023)
-
Application of mesenchymal stem cells for anti-senescence and clinical challenges
Stem Cell Research & Therapy (2023)
-
Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases
Signal Transduction and Targeted Therapy (2023)