Abstract
Selective defluorinative functionalization of trifluoromethyl group (–CF3) is an attractive synthetic route to the pharmaceutically privileged fluorine-containing moiety. Herein, we report a strategy based on photoexcited copper catalysis to activate the C–F bond of di- or trifluoromethylated arenes for divergent radical C–N coupling with carbazoles and aromatic amines. The use of different ligands can tune the reaction products diversity. A range of substituted, structurally diverse α,α-difluoromethylamines can be obtained from trifluoromethylated arenes via defluorinative C-N coupling with carbazoles, while an interesting double defluorinative C-N coupling is ready for difluoromethylated arenes. Based on this success, a carbazole-centered PNP ligand is designed to be an optimal ligand, enabling a copper-catalyzed C–N coupling for the construction of imidoyl fluorides from aromatic amines through double C-F bond functionalization. Interestingly, a 1,2-difluoroalkylamination strategy of styrenes is also developed, delivering γ,γ-difluoroalkylamines, a bioisostere to β-aminoketones, in synthetically useful yields. The DFT studies reveal an inner-sphere electron transfer mechanism for Cu-catalyzed selective activation of C(sp3)–F bonds.
Similar content being viewed by others
Introduction
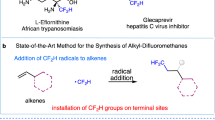
The unique physical and biological properties of fluorinated compounds have led to their wide application in pharmaceuticals1, agrochemicals2 and materials3. With their high lipophilicity, metabolic stability and unique electronic features, CF2 groups have attracted increasing attention (Fig. 1a)4. In recent decades, deoxyfluorination5,6, site-selective fluorination7,8,9 and fluoroalkylation10,11,12,13,14,15 reactions have been used to construct this interesting motif. Meanwhile, selective functionalization of one C–F bond in trifluoromethyl groups has also gained great momentum because it can provide elegant access to a series of privileged compounds derived from commercially available trifluoromethylated arenes16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. Recently, radical defluoroalkylation34,35,36, defluoroarylation37, defluorohydrogenation38,39,40 and defluorocarboxylation32 reactions have been achieved, all of which constructed C–C or C–H bonds from C–F bonds in a trifluoromethyl group. A very recent work from Xu’s group disclosed a radical coupling pathway for the formation of C–X (X = O, S, Se) bonds with reactive Ar-XH (pKa ~6)41. However, to the best of our knowledge, catalytic defluorinative C–N coupling from C–F bond in trifluoromethylated arenes has been reported very rarely (Fig. 1b)25,42,43,44.
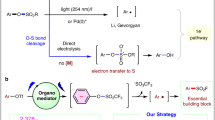
a The prevalence of CFn-N unit in biologically and chemically important molecules. b Selective functionalization of the C(sp3)-F bond in Ar-CF3. c The state-of-the-art for copper-catalyed C(sp3)-N coupling. d This work: photoinduced copper-catalyzed divergent C–N coupling of di- or trifluoromethyl arenes.
Remarkably, α,α-difluoromethylamines are bioisosteres for amides45, and this would be significant in new drug discovery. Several issues, however, have hindered the construction of such structures and these include: (1) the bond dissociation energy (BDE) of C–F bond in CF3 is strong46 but the BDEs of the remaining C–F bonds significantly decreases once the F atoms have been substituted. For example, the BDEs of the C–F bonds in PhCF3, PhCHF2, and PhCH2F are 118, 107 and 99 kcal mol−1, respectively47, which often contributes to undesired over-defluorination; (2) the reactions of the difluoromethyl radical are generally limited to radical addition or a HAT process and thus construction of CF2–X bond calls for merging strategies such as the combination of transition metal catalysis. For example, Zhang and co-workers have reported an elegant photoinduced Pd-catalyzed defluorinative arylation, and the combination of transition metal catalysis unlocked new reaction pathways for selective functionalization of C(sp3)–F bond37. In recent years, Cu-mediated radical coupling with light excitation has become an emerging field48,49. A series of pioneering copper-catalyzed C–N couplings via a radical pathway using Ar–X (X = Cl, Br, I)50,51 or activated aliphatic halides (R–X, X = Cl, Br, I)52,53,54,55,56,57,58,59 has been disclosed by Fu and Liu respectively. However, the activation of more inert C–F bonds56 to construct C–N bonds is still very challenging (Fig. 1c). Inspired by these seminal works, we wondered if photoinduced copper-catalyzed defluorinative C–N coupling of a CF3 group could afford difluoromethylated products, whose synthesis otherwise would be difficult.
Here, we report copper-catalyzed divergent defluorinative C–N coupling of trifluoromethylated arenes with carbazoles and aromatic amines. A photoexcited copper-complex can accomplish the inner-sphere electron transfer with the C–F bond in Ar-CF3 to generate the difluoromethyl radical as key intermediates (Fig. 1d). These radicals can interact with the generated R2N-CuII-species for radical C–N coupling. An interesting double defluorinative C–N coupling has been realized for difluoromethylated arenes. When primary aromatic amines are used as the nitrogen source with a carbazole-centered PNP ligand, a continuous defluorination process would occur to afford versatile imidoyl fluorides in synthetically useful yields. In addition, 1,2-difluoroalkylamination of styrenes can be realized to furnish useful β-aminoketones bioisosteres, γ,γ-difluoroalkylamines. This protocol offers a divergent C–N coupling for the synthesis of synthetically interesting α,α-difluoromethylamines, imidoyl fluorides and γ,γ-difluoroalkylamines from electron-deficient trifluoromethylated arenes and aromatic amines. The broad reaction scope, excellent functional group tolerance and gram-scale ability enable this strategy to be promising for the construction of value-added products.
Results
Reaction optimization
To initiate this study, the defluorinative C–N coupling reaction of 1,3-bis(trifluoromethyl)-benzene (1a) and carbazole (2a) was selected as the model reaction with which to optimize the reaction conditions (Table 1). The standard conditions include the use of CuBr as catalyst and nBu3P as the ligand with tBuOLi as an inorganic base under LED irradiation (λmax = 390 nm). This delivered the desired defluorinated product (3a) in 72% isolated yield in 1 h (Table 1, entry 1). It was found that the replacement of tBuOLi with tBuOK resulted in only a trace amount of product (entry 2). It was considered that tBuOLi may have better solubility in methyl tert-butyl ether (MTBE) and also that the lithium ion could be a hard acid to facilitate the elimination of the fluoride60. A series of different solvents were examined and it was found that ethers, such as diethyl ether and methyl tert-butyl ether gave better yields (entries 3 and 4). When CuCl replaced CuBr (entry 5), a comparable yield of 72% was obtained but CuI as catalyst furnished the product (3a) in only 14% yield (entry 6). In the absence of nBu3P as ligand, a decreased yield of 13% was obtained (entry 7). The control experiments showed that this reaction cannot occur in the absence of either the catalyst, CuBr or light irradiation (entries 8 and 9).
Substrate scope
With the standard reaction conditions in hand, we investigated the scope of substrates. As shown in Fig. 2, a variety of trifluoromethyl arenes can afford the desired C–N coupling products (3a–3s) in moderate to good yields. A series of synthetically useful functional groups, such as chloride (3e), terminal alkene (3h), silane (3i), alkyne (3l), thioether (3n), nitrile (3q) and ester (3r) tolerate the reaction conditions well. The gram-scale experiment was also carried out without compromising the reaction efficiency and the product (3h) was obtained in 52% isolated yield at 5 mmol scale. When the trifluoromethylated arenes bearing a triarylphosphine moiety were subjected to the standard conditions, the reaction was not affected and the desired product (3j) was obtained in 50% yield. Heterocyclic substrates such as trifluoromethyl pyridines can also give corresponding products (3o, 3p) in moderate yields albeit with an increased catalyst loading (10 mol%) and longer reaction time (12 h). Interestingly, when the arenes bearing an electron-withdrawing group at the para-position relative to the trifluoromethyl group were employed, the alkylphosphine ligand (nBu3P) failed to promote the desired coupling reaction. We speculated that electron-rich ligands are disfavored to the crucial coupling process, thus hindering the formation of products37,61. Importantly, an electron-deficient ligand can accelerate this process. When an electron-poor phosphite ester ligand, P(OAr)3 (Ar = 2,4-tBu2C6H3) was used in place of nBu3P, the desired products (3q, 3r) were formed in moderate yields. When benzotrifluorides was subjected to this protocol, the product (3s) could only be obtained in 17% isolated yield. In addition, α-(trifluoromethyl)styrene is also a suitable substrate to deliver the target product (3t) in good selectivity.
Subsequently, the scope of the nitrogen-containing partners was investigated. It was found that the carbazoles bearing various functional groups such as methoxy (3w), amine (3x), fluoride (3y), N-, O-, S-containing heteroaromatics (3z, 3aa, 3bb), chloride (3dd), epoxide (3ee) and alkene (3gg) all gave the desired products in moderate to good yields. When multisubstituted indole was subjected to the reaction, the product (3hh) was produced in only 15% yield. Unfortunately, anilines cannot successfully react in copper-catalyzed defluorinative C–N coupling. We rationalized that the conjugated system in carbazole would facilitate the excitation process of the formed [L-Cu-N-carbazole] species41, thus promoting the desired defluorinative C–N coupling.
Interestingly, the difluoromethylated arenes are also good coupling partners in Cu-catalyzed defluorinative C–N coupling. Because the difference between the difluoro- and monofluoro-variants, double defluorinative C–N coupling processes could take place for difluoromethylated arenes. Following such reactions, the desired products (3ii–3ll) were obtained in yields of 60–73%. We speculated the second C–N formation could experience either the radical pathway via the α-N-benzylic radical or the ionic mechanism via the iminium ions. Importantly, this protocol can be applied to the late-stage modification of complex trifluoromethylated arenes and carbazoles. As shown in Fig. 2, the Aprepitant precursor, the Fluoxetine derivative and the carbazole alkaloid, Murrayafoline A are good substrates, which can afford the corresponding products (3mm–3oo) in synthetically useful yields.
Mechanistic studies
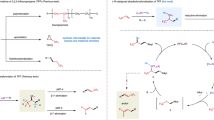
To gain further insight into the reaction mechanism, control experiments were conducted and the results are shown in Fig. 3. To confirm whether a radical process is involved or not, 2,2,6,6-Tetramethylpiperidinooxy (TEMPO) was added into the model reaction (Fig. 3a). As expected, the reaction was completely inhibited and TEMPO-trapped difluorobenzylic radical adduct (5) was obtained with 45% isolated yield (Eq. 1). When 1.5 equiv. of (1-cyclopropylvinyl)benzene (6) was added to the model reaction under standard conditions, it was found that the desired product (3a) was formed in 12% yield and the radical ring-opening product (7) was detected in 26% NMR yield (Eq. 2). Thus, it demonstrates that the reaction proceeds with a radical pathway. The wavelength of experimental LEDs ranged from 360 to 420 nm with λmax = ~390 nm (Supplementary Fig. 4). To identify the light-absorbing species in the catalytic cycle, UV-Vis absorption experiments were conducted. It indicated that trifluoromethylated arenes (1a), carbazole (2a), CuBr, CuBr/nBu3P or 2a/tBuOLi all have very weak absorptions in the range of 360–420 nm, while the mixture of CuBr, nBu3P, carbazole (2a) and tBuOLi has significant UV-Vis absorption with two new peaks at 373 and 393 nm (Fig. 3b). Consequently, we speculated that the [L-Cu-N-carbazole] species generated in-situ would absorb the light, and then promote the single electron reduction of trifluoromethylated arenes. Luminescence quenching experiments further confirmed that photoexcited [L-Cu-N-carbazole]* could be quenched by 1,3-bis(trifluoromethyl)-benzene (1a) (Fig. 3c). Light turn-on/off control experiments suggested that the reaction requires continuous irradiation of light, and almost no conversion occurred in the absence of light (Fig. 3d). On the other hand, the plots of kinetic experiments suggested a first-order kinetic dependence on [Cu] and [1a] but a zero-order kinetic dependence on [2a] (Fig. 3e). These mechanistic results indicate that carbazole unit would play a crucial role in this single electron transfer process between photoexcited [L-CuI-N]* species and trifluoromethylated arenes. Inspired by this, we wondered if we could investigate one phosphine-based ligand bearing one carbazole moiety, which may further extend the N-sources in such defluorinative transformations.
In the light of recent achievements of PNP ligand53,62,63,64,65, we assumed that this skeleton combined with carbazoles and trialkyl phosphines would facilitate the desired defluorinative C–N coupling reaction and further expand the scope of amine substrates. Consequently, carbazole-centered PNP ligand (L1) was introduced to achieve copper-catalyzed C–N coupling of trifluoromethylated arenes and anilines. To our surprise, the resulting α,α-difluoromethylamines was immediately transformed to imidoyl fluorides through tandem C–N coupling and defluorination under basic conditions (Supplementary Table 10)66. Despite of the promising synthetic potential of imidoyl fluorides, their synthesis is challenging and in general harsh reaction conditions are required while with limited scope67,68,69,70. A series of structurally diverse imidoyl fluorides (9a–9n) can be readily obtained in synthetically useful yields from commercially available trifluoromethylated arenes and aromatic amines as shown in Fig. 4. A 10 mmol scale experiment was also tested and imidoyl fluorides (9b) can be obtained in 57% isolated yield, further demonstrating its robust synthetic practicality. It is worth noting that aniline with halogen such as bromide (9i) was also compatible with this reaction. The heteroaromatic amines (9n) also tolerated this reaction conditions well. When secondary amines such as N-methylaniline was employed as the N-based nucleophile, the desired defluorinative C–N coupling product (9o) could be detected with ~5% yield by GC-MS.
Provided by the unique reactivity of α,α-difluorobenzylic radical intermediate in the defluorinative C–N coupling process, we questioned if alkenes could be introduced into the reaction system to achieve 1,2-difluoroalkylamination reaction of the alkenes. Once successful, it can afford a new route for the synthesis of γ,γ-difluoroalkylamines, one of the bioisosteres to β-aminoketones. With this consideration, several alkenes were investigated and styrenes were found to be the most suitable radical receptor (Supplementary Table 17). For the aliphatic alkenes, only trace amount of 1,2-difunctionalization products can be detected and the formation of imidoyl fluorides (9) remains the main reaction process. We rationalized that the lower stability of corresponding alkyl radical, which may increase the energy barrier to interact with copper-species71. A wide range of useful functional groups such as silane (11c), chloride (11d and 11j), bromide (11e and 11k), methoxy (11i) were found to be tolerated well, producing expected 1,2-difunctionalized products in moderate yields as shown in Fig. 5. Ortho-substituted styrenes (11 m) had little influence on the yield. Besides, 1,1-disubstituted olefins (11n and 11o), internal alkenes and electron-withdrawing alkene were also suitable substrates to furnish products (11p and 11q) in moderate yields. In addition, it is interesting to find that phenylhydrazines can proceed this 1,2-difluoroalkylamination to deliver the expected product (11r) in 42% yield.
Synthetic application
To further explore the synthetic utility of this reaction, Ni-catalyzed remote migratory hydroarylation and epoxidation with 3-chloroperoxybenzoic acid (m-CPBA) of 3h gave the target products (12, 13) in 68 and 88% yields, respectively (Fig. 6a). As to imidoyl fluoride 9b, the corresponding products (14, 15) can be obtained in 91 and 89% yields through the substitution of methoxide and the cyclization reaction of tetrabutylammonium azides. The defluorinative C–N coupling strategy was used to introduce a difluoromethyl unit into the antihypertensive drug carvedilol. As shown in Fig. 6b, product (16) can be obtained by stepwise defluorinative C–N coupling and subsequent nucleophilic ring-opening reaction of 2-(2-methoxyphenoxy)ethan-1-amine to the epoxide (3ee).
Theoretical study
A possible mechanism is proposed in Fig. 7a. Upon light irradiation, the catalyst [L1CuI] (17) is excited to [L1CuI]* species (18), which can undergo either an outer-sphere single electron transfer (OSET) process with the highly electron-deficient trifluoromethylated arene (1) to furnish the [L1CuII]+ intermediate (19) and a radical anion (20) or an inner-sphere single electron transfer (ISET) to furnish [L1CuII-F] (21) and the difluorobenzylic radical (22) directly. The radical anion (20) from the OSET process would lose one fluoride to produce difluorobenzylic radical (22). When styrenes are employed, 22 is easily converted to the more stable secondary benzyl radical (23). Through the ligand substitution process, [L1CuII-F] (21) can be transformed to [L1CuII-NHAr] species (24), which could interact with the difluorobenzylic radical (22) or the benzyl radical (23) to give the coupling products 3 or 11, respectively. Although the difluorobenzylic radical (22) or the benzyl radical (23) is less likely to proceed radical oxidative addition to generate Cu(III)-intermediate for subsequent reductive elimination55,56, its possibility to generate coupling products (3 or 11) cannot be completely ruled out72. Specially, NH in the α,α-difluoromethylamines (3) becomes much more acidic with the influence of a difluoromethyl group, and thus the easy elimination of one fluoride would give rise to imidoyl fluoride (9) as the final product.
Based on our recent theoretical understanding of the ISET process73.74 DFT calculations were performed to elucidate the crucial SET process in the proposed mechanism. All computational calculations were carried out by Gaussian 09B (see computational details in Supplementary Information Section 8). As depicted in Fig. 7b, ISET can facilitate one-step generation of [L1CuII-F] (21) and α,α-difluorobenzylic radical (22), featuring a low energy barrier of only 5.8 kcal∙mol-1 (TS1), accompanied with a free energy drop of -13.2 kcal∙mol-1. Notably, in the precursor for ISET (INT1) at triplet state, π-π stacking effect between the PNP ligand and the electron-deficient trifluoromethylated arenes (1) facilitates the substrate-catalyst binding between L1Cu and 1, thus promoting the crucial electron transfer process (Supplementary Fig. 16).
On the other hand, the OSET pathway involved stepwise electron transfer and the dissociation process of the C–F bond. The free energy barrier for the electron transfer was estimated to be 10.7 kcal∙mol-1, which is higher than that with ISET process. Consequently, the ISET pathway exhibited superior kinetic favorability over the OSET one. It would enrich copper-catalyzed electron transfer mechanism. Further computational analyses were also performed to explore potential pathways of C–N cross-coupling (Supplementary Fig. 16). These calculations indicated that the reaction favored the ISET pathway, followed by MECP (Minimum Energy Crossing-Point)75 mediated radical capturing (RC) process.
Discussion
In summary, we have developed a divergent defluorinative C–N coupling reaction of electron-deficient di- and trifluoromethylated arenes and aromatic amines via photoexcited copper catalysis. Highly selective C–N coupling, tandem C–N coupling/defluorination and 1,2-difluoroalkylamination of styrenes have been readily realized under mild reaction conditions. This protocol can afford a variety of structurally diverse α,α-difluoromethylamines, imidoyl fluoride and γ,γ-difluoroalkylamines with good functional group tolerance. DTF studies of the mechanism identify the difluorobenzylic radicals produced via inner-sphere electron transfer as the key intermediates for the subsequent C–N coupling process. This protocol is a progressive step in copper-catalyzed inert C–F bond activation. It should also represent an important step forward to divergent transformations of trifluoromethylated compounds into privileged fluorine-containing moiety.
Methods
General procedure for defluorinative C–N coupling of carbazoles
An oven-dried vial (8 mL) equipped with a magnetic stir bar, is charged with CuBr (0.29 mg, 0.002 mmol, 2 mol%), carbazole 2 (0.1 mmol, 1 equiv.), tBuOLi (16.0 mg, 0.2 mmol, 2 equiv.), MTBE (4 mL) and nBu3P (1.2 µL, 0.0048 mmol, 4.8 mol%). The resulting reaction mixture is allowed to stir at rt for 30 min. Subsequently, the trifluoromethylated arene 1 (0.5 mmol, 5 equiv.) is added. Then the vial is sealed with a rubber cap and is irradiated under 390 nm LEDs for 1 h at rt with vigorous stirring. When the reaction is completed, the mixture is concentrated in vacuo. The crude product can be purified by flash column chromatography on neutral Al2O3 with petroleum ether, ethyl acetate and triethylamine as eluent to afford the desired coupling products.
General procedure for the tandem C–N coupling and defluorination
An oven-dried vial (4 mL) equipped with a magnetic stir bar, is charged with CuBr (1.43 mg, 0.01 mmol, 5 mol %), L1 (7.0 mg, 0.01 mmol, 5 mol %), tBuOLi (48.0 mg, 0.6 mmol, 3 equiv.) and Et2O (2 mL). The resulting reaction mixture is allowed to stir at rt for 30 min. Subsequently, the trifluoromethylated arene 1 (0.6 mmol, 3 equiv.) and aromatic amine 8 (0.2 mmol, 1 equiv.) are added sequentially. Then the vial is sealed with a rubber cap and is irradiated under 390 nm LEDs for 2 h at rt with vigorous stirring. When the reaction is completed, the mixture is concentrated in vacuo. The crude product can be purified by flash column chromatography on neutral Al2O3 with petroleum ether and ethyl acetate as eluent to afford the desired coupling products.
General procedure for the 1,2-difluoroalkylamination reaction
An oven-dried vial (4 mL) equipped with a magnetic stir bar, is charged with CuBr (1.43 mg, 0.01 mmol, 5 mol %), L1 (7.0 mg, 0.01 mmol, 5 mol %), tBuOLi (48.0 mg, 0.6 mmol, 3 equiv.) and Et2O (2 mL). The resulting reaction mixture is allowed to stir at rt for 30 min. Subsequently, the trifluoromethylated arene 1 (0.6 mmol, 3 equiv.), styrene 10 (0.6 mmol, 3 equiv.) and aromatic amine 8 (0.2 mmol, 1 equiv.) are added sequentially. Then the vial is sealed with a rubber cap and is irradiated under 390 nm LEDs for 2 h at rt with vigorous stirring. When the reaction is completed, the mixture is concentrated in vacuo. The crude product can be purified by flash column chromatography on silica gel with petroleum ether and ethyl acetate as eluent to afford the desired coupling products.
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2212746 (3u), CCDC 2270870 (catalyst L1Cu), and CCDC 2281146 (9b). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Data related to materials and methods, optimization of conditions, experimental procedures, mechanistic experiments, and spectra are provided in the Supplementary Information. Source data containing the coordinates of the optimized structures are present. All data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Fujiwara, T. & O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 167, 16–29 (2014).
Berger, R. et al. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40, 3496–3508 (2011).
Ni, C. & Hu, J. The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 45, 5441–5454 (2016).
Middleton, W. J. & Bingham, E. M. α,α-Difluoroarylacetic acids: preparation from (diethylamino)sulfur trifluoride and α-oxoarylacetates. J. Org. Chem. 45, 2883–2887 (1980).
Ni, C., Hu, M. & Hu, J. Good partnership between sulfur and fluorine: sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 115, 765–825 (2015).
Xia, J.-B., Zhu, C. & Chen, C. Visible light-promoted metal-free C–H activation: diarylketone-catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc. 135, 17494–17500 (2013).
Xu, P., Guo, S., Wang, L. & Tang, P. Silver-catalyzed oxidative activation of benzylic C-H bonds for the synthesis of difluoromethylated arenes. Angew. Chem. Int. Ed. 53, 5955–5958 (2014).
Ning, Y. et al. Synthesis of β-difluoroalkyl azides via elusive 1,2-azide. Migr. Chem. 6, 486–496 (2020).
Feng, Z., Xiao, Y.-L. & Zhang, X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 51, 2264–2278 (2018).
Ma, X. & Song, Q. Recent progress on selective deconstructive modes of halodifluoromethyl and trifluoromethyl-containing reagents. Chem. Soc. Rev. 49, 9197–9219 (2020).
Xie, J. et al. Gold-catalyzed highly selective photoredox C(sp2)−H difluoroalkylation and perfluoroalkylation of hydrazones. Angew. Chem. Int. Ed. 55, 2934–2938 (2016).
Fu, X.-P. et al. Controllable catalytic difluorocarbene transfer enables access to diversified fluoroalkylated arenes. Nat. Chem. 11, 948–956 (2019).
Zhang, X. et al. Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines. Nature 594, 217–222 (2021).
Feng, X.-T. et al. 3,3-difluoroallyl sulfonium salts: practical and bench-stable reagents for highly regioselective gem-difluoroallylations. Angew. Chem. Int. Ed. 61, e202210103 (2022).
Jaroschik, F. Picking one out of three: selective single C−F activation in trifluoromethyl groups. Chem. Eur. J. 24, 14572–14582 (2018).
Gupta, R., Jaiswal, A. K., Mandal, D. & Young, R. D. A frustrated Lewis pair solution to a frustrating problem: mono-selective functionalization of C–F bonds in di- and trifluoromethyl groups. Synlett 31, 933–937 (2020).
Yan, G., Qiu, K. & Guo, M. Recent advance in the C–F bond functionalization of trifluoromethyl-containing compounds. Org. Chem. Front. 8, 3915–3942 (2021).
Ai, H.-J., Ma, X., Song, Q. & Wu, X.-F. C-F bond activation under transition-metal-free conditions. Sci. China Chem. 64, 1630–1659 (2021).
Carvalho, D. R. & Christian, A. H. Modern approaches towards the synthesis of geminal difluoroalkyl groups. Org. Biomol. Chem. 19, 947–964 (2021).
An, X. et al. Recent advances in the single C—F bond cleavage reactions of trifluoromethylarenes. Chin. J. Org. Chem. 41, 4554–4564 (2021).
Zhao, F., Zhou, W. & Zuo, Z. Recent advances in the synthesis of difluorinated architectures from trifluoromethyl groups. Adv. Synth. Catal. 364, 234–267 (2022).
Simur, T. T. et al. C–F bond functionalizations of trifluoromethyl groups via radical intermediates. Chin. Chem. Lett. 33, 1193–1198 (2022).
Yoshida, S., Shimomori, K., Kim, Y. & Hosoya, T. Single C−F bond cleavage of trifluoromethylarenes with an ortho-silyl group. Angew. Chem. Int. Ed. 55, 10406–10409 (2016).
Mandal, D., Gupta, R., Jaiswal, A. K. & Young, R. D. Frustrated Lewis-pair-meditated selective single fluoride substitution in trifluoromethyl groups. J. Am. Chem. Soc. 142, 2572–2578 (2020).
Wright, S. E. & Bandar, J. S. A base-promoted reductive coupling platform for the divergent defluorofunctionalization of trifluoromethylarenes. J. Am. Chem. Soc. 144, 13032–13038 (2022).
Zhou, F.-Y. & Jiao, L. Asymmetric defluoroallylation of 4-trifluoromethylpyridines enabled by umpolung C−F bond activation. Angew. Chem. Int. Ed. 61, e202201102 (2022).
Chen, K., Berg, N., Gschwind, R. & König, B. Selective single C(sp3)–F bond cleavage in trifluoromethylarenes: merging visible-light catalysis with lewis acid activation. J. Am. Chem. Soc. 139, 18444–18447 (2017).
Wang, H. & Jui, N. T. Catalytic defluoroalkylation of trifluoromethylaromatics with unactivated alkenes. J. Am. Chem. Soc. 140, 163–166 (2018).
Luo, C. & Bandar, J. S. Selective defluoroallylation of trifluoromethylarenes. J. Am. Chem. Soc. 141, 14120–14125 (2019).
Campbell, M. W. et al. Photochemical C–F activation enables defluorinative alkylation of trifluoroacetates and -acetamides. J. Am. Chem. Soc. 143, 19648–19654 (2021).
Yan, S.-S. et al. Visible-light photoredox-catalyzed selective carboxylation of C(sp3)−F bonds with CO2. Chem 7, 3099–3113 (2021).
Wang, J. et al. Late-stage modification of drugs via alkene formal insertion into benzylic C−F bond. Angew. Chem. Int. Ed. 62, e202215062 (2023).
Yu, Y.-J. et al. Sequential C-F bond functionalizations of trifluoroacetamides and acetates via spin-center shifts. Science 371, 1232–1240 (2021).
Liu, C., Shen, N. & Shang, R. Photocatalytic defluoroalkylation and hydrodefluorination of trifluoromethyls using o-phosphinophenolate. Nat. Commun. 13, 354 (2022).
Ye, J.-H., Bellotti, P., Heusel, C. & Glorius, F. Photoredox-catalyzed defluorinative functionalizations of polyfluorinated aliphatic amides and esters. Angew. Chem. Int. Ed. 61, e202115456 (2022).
Luo, Y.-C. et al. Visible-light-induced palladium-catalyzed selective defluoroarylation of trifluoromethylarenes with arylboronic acids. J. Am. Chem. Soc. 143, 13971–13979 (2021).
Munoz, S. B. et al. Selective late-stage hydrodefluorination of trifluoromethylarenes: a facile access to difluoromethylarenes. Eur. J. Org. Chem. 2017, 2322–2326 (2017).
Sap, J. B. I. et al. Organophotoredox hydrodefluorination of trifluoromethylarenes with translational applicability to drug discovery. J. Am. Chem. Soc. 142, 9181–9187 (2020).
Shen, Z.-J. et al. Organoboron reagent-controlled selective (deutero)hydrodefluorination. Angew. Chem. Int. Ed. 62, e202217244 (2023).
Xu, J. et al. Construction of C–X (X = S, O, Se) bonds via Lewis acid-promoted functionalization of trifluoromethylarenes. ACS Catal. 13, 7339–7346 (2023).
Mkrtchyan, S. et al. Mechanochemical transformation of CF3 group: synthesis of amides and Schiff bases. Adv. Synth. Catal. 363, 5448–5460 (2021).
Khanapur, S. et al. Fluorine-18 labeling of difluoromethyl and trifluoromethyl groups via monoselective C−F bond activation. Angew. Chem. Int. Ed. 61, e202210917 (2022).
Gupta, R., Csókás, D., Lye, K. & Young, R. D. Experimental and computational insights into the mechanism of FLP mediated selective C–F bond activation. Chem. Sci. 14, 1291–1300 (2023).
Zafrani, Y. et al. Difluoromethyl bioisostere: examining the “lipophilic hydrogen bond donor” concept. J. Med. Chem. 60, 797–804 (2017).
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
Luo, Y. R. Comprehensive Handbook of Chemical Bond Energies 1st edn, 211–217 (CRC Press, 2007).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Beaudelot, J. et al. Photoactive copper complexes: properties and applications. Chem. Rev. 122, 16365–16609 (2022).
Creutz, S. E., Lotito, K. J., Fu, G. C. & Peters, J. C. Photoinduced Ullmann C–N coupling: demonstrating the viability of a radical pathway. Science 338, 647–651 (2012).
Sagadevan, A. et al. Visible-light copper nanocluster catalysis for the C–N coupling of aryl chlorides at room temperature. J. Am. Chem. Soc. 144, 12052–12061 (2022).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Ahn, J. M., Peters, J. C. & Fu, G. C. Design of a photoredox catalyst that enables the direct synthesis of carbamate-protected primary amines via photoinduced, copper-catalyzed N-alkylation reactions of unactivated secondary halides. J. Am. Chem. Soc. 139, 18101–18106 (2017).
Matier, C. D., Schwaben, J., Peters, J. C. & Fu, G. C. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 139, 17707–17710 (2017).
Lee, H. et al. Investigation of the C–N bond-forming step in a photoinduced, copper-catalyzed enantioconvergent N–alkylation: characterization and application of a stabilized organic radical as a mechanistic probe. J. Am. Chem. Soc. 144, 4114–4123 (2022).
Cho, H. et al. Photoinduced, copper-catalyzed enantioconvergent alkylations of anilines by racemic tertiary electrophiles: synthesis and mechanism. J. Am. Chem. Soc. 144, 4550–4558 (2022).
Zhang, Y.-F. et al. Enantioconvergent Cu-catalyzed radical C–N coupling of racemic secondary alkyl halides to access α-chiral primary amines. J. Am. Chem. Soc. 143, 15413–15419 (2021).
Dong, X.-Y., Li, Z.-L., Gu, Q.-S. & Liu, X.-Y. Ligand development for copper-catalyzed enantioconvergent radical cross-coupling of racemic alkyl halides. J. Am. Chem. Soc. 144, 17319–17329 (2022).
Chen, J.-J. et al. Enantioconvergent Cu-catalyzed N-alkylation of aliphatic amines. Nature https://doi.org/10.1038/s41586-023-05950-8 (2023).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Hou, Z. et al. One-electron reduction of aromatic ketones by low-valent lanthanides. isolation, structural characterization, and reactivity of lanthanide ketyl complexes. J. Am. Chem. Soc. 120, 754–766 (1998).
Morales-Morales, D. & Jensen, C. M. (ed.) The Chemistry of Pincer Compounds (Elsevier, 2007).
Koten, G. V. Pincer ligands as powerful tools for catalysis in organic synthesis. J. Organomet. Chem. 730, 156–164 (2013).
Peris, E. & Crabtree, R. H. Key factors in pincer ligand design. Chem. Soc. Rev. 47, 1959–1968 (2018).
Ott, J. C., Bürgy, D., Guan, H. & Gade, L. H. 3d metal complexes in T-shaped geometry as a gateway to metalloradical reactivity. Acc. Chem. Res. 55, 857–868 (2022).
Chen, Y.-L. et al. Dual role of (NH4)2CO3 enables defluorinative synthesis of β-fluoroalkylated aminovinyl ketones. Org. Lett. 24, 9086–9091 (2022).
Abramovitch, R. A., Pilski, J., Konitz, A. & Tomasik, P. Direct acylamination of pyridine 1-oxide with N-phenylarenimidoyl chlorides and fluorides. J. Org. Chem. 48, 4391–4393 (1983).
Guo, R., Zhang, Z., Shi, F. & Tang, P. Tunable cascade reaction of aryl diazonium salts and trialkylamine: synthesis of monofluorinated arylhydrazones and gem-difluorinated azo compounds. Org. Lett. 18, 1008–1011 (2016).
Weidlich, F. et al. Mild C-F activation in perfluorinated arenes through photosensitized insertion of isonitriles at 350nm. Adv. Synth. Catal. 362, 376–38 (2020).
Kim, H. E., Choi, J.-H. & Chung, W.-J. Fluorine-assisted rearrangement of geminal azidofluorides to imidoyl fluorides. J. Org. Chem. 88, 6878–6889 (2023).
Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)–H functionalization. Chem. Soc. Rev. 51, 1640–1658 (2022).
Casitas, A. & Ribas, X. The role of organometallic copper(III) complexes in homogeneous catalysis. Chem. Sci. 4, 2301–2318 (2013).
Ji, C.-L. et al. Photoinduced gold-catalyzed divergent dechloroalkylation of gem-dichloroalkanes. Nat. Catal. 5, 1098–1109 (2022).
Fang, Q.-Y. et al. Trinuclear gold-catalyzed 1,2-difunctionalization of alkenes. Angew. Chem. Int. Ed. 62, e202305121 (2023).
Schröder, D., Shaik, S. & Schwarz, H. Two-state reactivity as a new concept in organometallic chemistry. Acc. Chem. Res. 33, 139–145 (2000).
Acknowledgements
We thank National Key Research and Development Program of China (2022YFA1503200 and 2021YFC2101901), the National Natural Science Foundation of China (22122103, 22101130, and 21971108), Fundamental Research Funds for the Central Universities (020514380304, 020514380252 and 020514380272) for financial support. All theoretical calculations were performed at the High-Performance Computing Center (HPCC) of Nanjing University. N.L., Y.L., and T.Z. at Nanjing University are gratefully acknowledged for their reproduction of the experimental procedures for products 3a, 3o, and 11n.
Author information
Authors and Affiliations
Contributions
J.X. and Jie Han conceived and designed the project. Jun Huang, T.Z., and W.L. performed and analyzed the experimental data. Q.G. performed the density functional theory calculations and discussed the results with S.C. and Jie Han, and J.X. co-wrote the manuscript with input from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Gao, Q., Zhong, T. et al. Photoinduced copper-catalyzed C–N coupling with trifluoromethylated arenes. Nat Commun 14, 8292 (2023). https://doi.org/10.1038/s41467-023-44097-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-44097-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.