Abstract
The size of plant lateral organs is determined by well-coordinated cell proliferation and cell expansion. Here, we report that miR159, an evolutionarily conserved microRNA, plays an essential role in regulating cell division in rose (Rosa hybrida) petals by modulating cytokinin catabolism. We uncover that Cytokinin Oxidase/Dehydrogenase6 (CKX6) is a target of miR159 in petals. Knocking down miR159 levels results in the accumulation of CKX6 transcripts and earlier cytokinin clearance, leading to a shortened cell division period and smaller petals. Conversely, knocking down CKX6 causes cytokinin accumulation and a prolonged developmental cell division period, mimicking the effects of exogenous cytokinin application. MYB73, a R2R3-type MYB transcription repressor, recruits a co-repressor (TOPLESS) and a histone deacetylase (HDA19) to form a suppression complex, which regulates MIR159 expression by modulating histone H3 lysine 9 acetylation levels at the MIR159 promoter. Our work sheds light on mechanisms for ensuring the correct timing of the exit from the cell division phase and thus organ size regulation by controlling cytokinin catabolism.
Similar content being viewed by others
Introduction
How the size of living organisms is regulated makes for a fascinating and attractive research question1,2,3. The final size of a given organ is carefully orchestrated by the complex integration of multiple intrinsic growth signals and external environmental cues. The growth of petals, just like other organs, goes through four phases from primordium to their final size: (1) founder cells are recruited to the primordium, (2) cell proliferation, (3) transition from cell proliferation to cell expansion, and (4) cell expansion. Final organ size is determined by a strict spatial and temporal coordination of these four events1,4. Organ size also reflects the integration of total cell number, which is determined by the number of cell divisions5,6,7,8 and the timing of proliferation arrest9,10,11, with cell size, which is defined by the duration of the cell expansion phase and the cell expansion rate1,12,13.
Several phytohormones, such as auxin, gibberellin (GA), cytokinins, and brassinosteroids (BRs), coordinately control the dynamics of the so-called “cell cycle arrest front”, which marks the transition from cell division to differentiation14,15,16,17,18. In Arabidopsis (Arabidopsis thaliana) leaves, most cells exit the division phase and begin expanding when the leaf blade reaches a certain size (about 10% of final size)19,20. ARGOS, encoded by an auxin-inducible gene, promotes cell proliferation by upregulating AINTEGUMENTA (ANT) and ANT-LIKE6 (AIL6) expression, leading to the transcriptional activation of the D-type cyclin gene CYCD3;111,21,22. AUXIN RESPONSE FACTOR8 (ARF8) interacts with the transcription factor BIG PETAL (BPEp) and synergistically restricts mitotic growth in petals by influencing proliferation and expansion23,24. Another auxin-responsive transcription factor, ARF2, suppresses the expression of ANT and CYCD3;1 and thus inhibits proliferation. Notably, ARF2 activity is itself repressed by protein phosphorylation via the brassinosteroid-responsive kinase BR-INSENSITIVE2 (BIN2)25,26, indicating that cross-talk between auxin and BRs contributes to cell proliferation and expansion. In parallel, GA induces the expression of STUNTED (STU), encoding a receptor-like cytoplasmic kinase that represses the expression of the cyclin-dependent kinase inhibitor genes SIAMESE (SIM) and SIAMESE-RELATED1 (SMR1), which encode pro-expansion factors by stimulating endoreduplication27. In maize (Zea mays) leaves, GA maintains cell proliferation, and a peak in GA concentration marks the boundary between cell proliferation and expansion zones15,28,29.
Cytokinins play vital roles in plant development by controlling cell division. Cytokinin metabolism and signal transduction are well understood30,31. Zeatin and isopentenyladenine-type cytokinins are considered to be the predominant forms of active cytokinins32,33. Steady-state levels of active cytokinins in a given organ are determined by the rates of biosynthesis and release of cytokinin nucleobase from their inactive conjugates and the rates of cytokinin degradation and inactivating conjugation30. Cytokinin oxidase/dehydrogenase (CKX) catalyzes the irreversible degradation of cytokinins34,35. In Arabidopsis, overexpressing CKX1 to CKX6 resulted in enhanced cytokinin breakdown and diminished activity of the vegetative and floral shoot apical meristems and leaf primordia36. Compared to the wild-type and single mutants, the ckx3 ckx5 double mutant produced larger flowers due to a delayed onset of cellular differentiation and an extended developmental cell division period within the inflorescence and floral meristems37.
microRNAs (miRNAs) are small RNAs of 20–24 nucleotides in length that play an essential role in developmental decisions38. Several miRNAs have been reported to control floral organ initiation, growth, and maturation. miR172 controls floral organ identity by binding to APETALA2 (AP2) mRNA and triggering its degradation or blocking APETALA2 protein translation in Arabidopsis39. miR319a governs petal and stamen development by targeting TCP4 mRNA encoding a member of the Teosinte branched1/Cincinnata/proliferating cell factor (TCP) family of transcription factors40. miR159, an evolutionarily conserved miRNA, is indispensable for normal floral organ growth41. The canonical miR159 target genes encode members of the GA-induced MYB (GAMYB) or GAMYB-like family, which are R2R3 MYB transcription factors present in most land plants and highly conserved42,43,44,45,46. Besides these conserved GAMYB targets, several other transcripts have been predicted to be potential miR159 targets in Arabidopsis, such as 1-AMINO-CYCLOPROPANE-1-CARBOXYLATE SYNTHASE8 (ACS8, At4g37770) and COPPER/ZINC SUPEROXIDE DISMUTASE3 (CSD3, At5g18100)47,48,49,50. In tomato (Solanum lycopersicum), miR159 regulates leaf and flower development by targeting SGN-U567133, a non-canonical target encoding a nucleus-localized protein with a NOZZLE-like domain51.
Our previous work predicted a CKX gene as a potential miR159 target in rose (Rosa hybrida)52. Here, we used rose petals as a model of lateral organ growth and discovered that miR159-mediated cytokinin clearance is required for controlling the duration of the cell division phase during the early petal growth period. Furthermore, we established that the MYB73–TOPLESS (TPL)–Histone Deacetylase19 (HDA19) repression complex developmentally determines MIR159 expression by modulating histone H3 lysine 9 acetylation (H3K9ac) levels at the MIR159 promoter. Importantly, mature miR159 targeted transcripts from the cytokinin oxidase/dehydrogenase gene RhCKX6 in petals for mRNA cleavage. Knockdown of miR159 abundance resulted in rapid accumulation of RhCKX6 transcripts, leading to cytokinin catabolism and a consequent shortened cell division period and smaller petals. Together, our results reveal a previously unknown level of regulatory complexity for ensuring the correct timing of cell division and organ size via controlling cytokinin catabolism.
Results
miR159 controls the duration of the cell division phase and flower development by targeting RhCKX6 transcripts in rose petals
Organ growth requires coordination between cell division and cell expansion53,54. During rose petal development, the adaxial and abaxial epidermal cells display distinct growth patterns. Adaxial cells continue to divide until the flower opens, making cell division a major contributor to adaxial petal area. Conversely, abaxial cells mostly stop division before bud opening, with cellular expansion becoming the primary contributor to abaxial petal area55,56,57. To understand the underlying control mechanism of cell division and cell expansion, we previously conducted a transcriptome deep sequencing (RNA-seq) analysis of short RNAs and transcripts from petals to investigate gene expression profiles during the cell division and expansion phases52,58. Based on our previous results, we defined stage 0 as “floral bud revealed” and stage 2 as “sepal completely unfolded”. Microscopy observations indicated that abaxial epidermal cell division almost stops at stage 2, whereas cell expansion drove petal growth from stage 2 onward, as demonstrated by the rapid increase of petal size at this stage (Supplementary Fig. 1).
The above dataset revealed that miR159 abundance exhibits a sharp drop in petals from cell division (stage 0) to cell expansion (5 days after stage 0)56,59,60. Small RNA blot and reverse transcription quantitative PCR (RT-qPCR) analyses confirmed the pattern of miR159 abundance in petals, with an huge drop from d 1 to d 5 after stage 0 (Fig. 1a; Supplementary Fig. 2a). The precursor sequence of this rose MIR159 (rhy-MIR159) shared high similarity with apple (Malus domestica) MIR159 (mdm-MIR159d/e/f), especially over the stem region (Supplementary Fig. 2b–d).
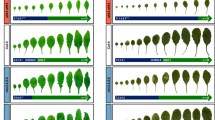
a Small RNA blot analysis of miR159 abundance in rose petals 1, 3, and 5 days after floral buds reached stage 0. Locked Nucleic Acids (LNA)-modified oligonucleotide probe was used to ensure the specificity of miR159. U6 was used as an internal control. The experiment was performed three times. b Reverse transcription quantitative PCR (RT-qPCR) analysis of miR159 levels in sepals (3 days after stage 0) of TRV and TRV-STTM159 plants. Data are shown as means ± SD (n = 4). 5 S rRNA was used as an internal control. Asterisks indicate statistically significant differences (two-sided Student’s t-test; ****P < 0.0001). c, d Flower opening progression of TRV and TRV-STTM159 plants. The experiments were performed independently twice with similar results, and one representative set of results is shown. Scale bars, 2 cm. Data are shown as means ± SD (n = 10). Asterisks indicate statistically significant differences (two-sided Student’s t-test; ***P < 0.001). e Petal size of TRV control and TRV-STTM159 plants at the fully opened stage. Data are shown as means ± SD (n = 3). The numbers below the images indicate petal size. Scale bar, 1 cm. Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05). f, g Cell number on the adaxial epidermis (f) and abaxial epidermis (g) in TRV control and TRV-STTM159 petals from 1 day after floral stage 0 to the fully opened stage. Data are shown as means ± SD (n = 3). Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05).
To characterize the function of miR159 in rose petals, we knocked down miR159 levels using a Tobacco rattle virus (TRV)-mediated short tandem target mimic (STTM) approach61,62 (Supplementary Fig. 3a). Notably, knocking down miR159 levels significantly shortened the petal cell division period (between stages 0 and 2), with 3.40 ± 0.80 days in TRV-STTM159 and 5.10 ± 0.70 days in the TRV control, while having no effect on the cell expansion period between stages 2 and 5 (Fig. 1b–d). The final petal size (at fully opened stage 5) of TRV-STTM159 lines was smaller than that of TRV controls (Fig. 1e). We recorded the number of adaxial and abaxial epidermal cells in TRV controls and TRV-STTM159 lines: knocking down miR159 levels resulted in a slower increase in the number of adaxial and abaxial epidermal cells, particularly in abaxial epidermal cells, than in control petals (Fig. 1f, g). These results indicate that the smaller petal size of TRV-STTM159 likely arose from fewer cells. Therefore, we hypothesized that miR159 affects the duration of cell proliferation in petals.
To explore the possible regulatory network involving miR159 during petal development, we compared the transcriptomes of TRV-STTM159 and TRV petals. We identified 1358 differentially expressed genes (DEGs) (using the criteria |Log2[fold change]| ≥ 1 and q-value ≤ 0.05 for TRV-STTM159/TRV), comprising 497 upregulated and 861 downregulated genes (Supplementary Fig. 3b; Supplementary Data 1). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of these DEGs indicated that terms associated with ‘plant hormone signal transduction’ and ‘zeatin biosynthesis’ are significantly enriched (Fig. 2a), suggesting that miR159 regulates phytohormone signaling, probably cytokinins. Notably, target prediction analysis identified 96 possible miR159 targets in the rose genome, including a CKX gene (RchiOBHmChr1g0319331) (https://www.zhaolab.org/psRNATarget/) (Supplementary Data 2 and Supplementary Fig. 4). Of all predicted targets, only RchiOBHmChr1g0319331 showed a robust increase in transcript levels in TRV-STTM159 lines compared to the TRV control, as would be expected for a true miR159 target (Fig. 2b–d). Moreover, RchiOBHmChr1g0319331 expression significantly increased from the cell division phase to the cell expansion phase, which was opposite to the pattern of miR159 abundance (Fig. 2e, f). Phylogenetic analysis showed that RchiOBHmChr1g0319331 encodes a CKX that is closely related to CKX6 from Arabidopsis; we thus named the rose gene RhCKX6 (Supplementary Fig. 5).
a Significantly enriched KEGG pathways in TRV-STTM159 lines. Arrows indicated the hormone-related pathways. b Venn diagram showing the extent of overlap between differentially expressed genes (DEGs) in TRV-STTM159 lines compared to TRV (absolute Log2[fold change] ≥ 1 and q-value ≤ 0.05) and predicted miR159-target genes. c, d Heatmap representation of transcript levels for possible miR159-targeted genes in TRV-STTM159 and TRV plants (c) and stage 0 and stage 2 (d). e, f RT-qPCR analysis of miR159 abundance (e) and RhCKX6 transcript levels (f) in rose petals at 1, 3, 5, and 7 days after stage 0. 5 S rRNA (e) or RhUBI2 (f) was used as an internal control. Data are shown as means ± SD (n = 3). Different lowercase letters indicate significant differences according to one-way ANOVA with Tukey’s multiple comparisons test (P < 0.05). g RT-qPCR analysis of RhCKX6 transcript levels in petals of TRV control and TRV-STTM159 at 1, 3, and 5 days after stage 0. RhUBI2 was used as an internal control. Data are shown as means ± SD (n = 3). Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05; **P < 0.01; ns no significant difference).
According to the target predictions above, the putative miR159 target site was located in the third exon of RhCKX6, 883–902 bp downstream of the translation start site of the open reading frame. We performed a 5′ RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) assay to test the miR159-dependent cleavage of RhCKX6 transcripts in rose petals. As shown in Fig. 3a, we mainly detected cleavage at several sites within the region complementary to miR159. We constructed RhCKX6-Sensor and RhCKX6m-Sensor constructs by cloning the coding sequence of the green fluorescent protein (GFP) gene in-frame with an intact miR159-target region (for RhCKX6-Sensor) or a mutated miR159-target region (for RhCKX6m-Sensor) (Fig. 3a, b). We then co-infiltrated each sensor construct in Nicotiana benthamiana leaves with a construct overexpressing the miR159 precursor (pre-MIR159) and a viral silencing suppressor p19; importantly, we detected lower GFP fluorescence for the pre-MIR159 + RhCKX6-Sensor combination compared to the sensor alone. By contrast, co-infiltrating pre-MIR159 with RhCKX6m-Sensor did not influence fluorescence intensity derived from RhCKX6m-Sensor (Fig. 3b, c). Immunoblot analysis confirmed the dependence of GFP abundance on miR159 (Fig. 3d). These results indicate that RhCKX6 is an authentic target of miR159 in rose. Furthermore, we detected a predicted miR159-target site in the putative homolog FvCKX6 from wild strawberry (Fragaria vesca). We observed miR159-mediated cleavage of FvCKX6 transcripts through RLM-RACE in strawberry petals and GFP-Sensor tests in N. benthamiana leaves, indicating that miR159-mediated cleavage is a common mechanism for regulating abundance of CKXs (Supplementary Fig. 6).
a Validation of miR159-targeted cleavage of RhCKX6. Upper panel, diagram of RhCKX6 mRNA. Red box, predicted cleavage site. Bottom panel, identification of cleavage sites using a 5′ RNA ligase-mediated rapid amplification of cDNA ends (5′ RLM-RACE) assay in rose petals. The positions of cleavage sites are indicated by arrows with the frequency of clones above. Dots represent Watson-Crick pairing. b, c Confocal imaging analysis (b) and relative fluorescence intensity (c) of N. benthamiana leaves 3 days after co-infiltrating pre-MIR159 with RhCKX6-sensor-GFP or RhCKX6m-sensor-GFP. Upper panel, diagrams of the constructs overexpressing pre-MIR159, RhCKX6-sensor-GFP, and RhCKX6m-sensor-GFP. Bottom panel, GFP fluorescence in N. benthamiana leaves 3 days after co-infiltrating the indicated constructs. Scale bars, 5 mm. The data in (c) are shown as means ± SD (n = 3). Asterisks indicate statistically significant differences (two-sided Student’s t-test; ***P < 0.001; ns, no significant difference). d Immunoblot analysis of GFP protein levels in N. benthamiana leaves 3 days after co-infiltrating pre-MIR159 with RhCKX6-sensor-GFP or RhCKX6m-sensor-GFP. Total proteins were extracted from leaves 3 days after infiltration and were subjected to immunoblot analysis using an anti-GFP antibody, with β-tubulin as an internal control. The experiments were performed independently three times, and representative results are shown (b–d).
Considering that GA-induced MYBs are conserved miR159 targets, we tested if the miR159–GAMYBs module contributes to cell division in petals. The three GAMYB members RhMYB33, RhMYB65, and RhMYB101 were predicted as miR159 targets in rose (Supplementary Fig. 4). RNA-seq and RT-qPCR analyses failed to detect RhMYB65 or RhMYB101 expression in rose petals at all tested development stages (1 d, 3 d, and 5 d after stage 0). RLM-RACE in rose petals and GFP-Sensor tests in N. benthamiana leaves demonstrated the miR159-dependent cleavage of RhMYB33 transcripts (Supplementary Fig. 7a, b). However, RhMYB33 expression showed a slight but non-significant decrease in petals from 3 d to 7 d after stage 0 (Supplementary Fig. 7c), concomitant with a significant drop in miR159 levels (Fig. 2e). In addition, we measured GA levels in rose petals. GA3 and GA7 levels were very low, whereas GA1 and GA19 levels were stable throughout the developmental time course (Supplementary Fig. 7d).
miR159 and miR319 exhibit high sequence similarity and share overlapping target sites63. In Arabidopsis, miR319 targets the transcription factor gene TCP4 to control floral organ morphology40. We thus tested whether miR319 is involved in regulating petal growth in rose. Small RNA blot and RT-qPCR analyses demonstrated that miR319 abundance is much lower than that of miR159, and remains relatively constant during the entire petal growth period (Supplementary Fig. 8a–c). The expression levels of three predicted miR319 targets (TCP2, TCP4, and TCP4-X1) did not change in miR159-knockdown lines (Supplementary Fig. 8d). Based on these findings, we propose that the regulation of petal growth through the miR319–TCPs module is not as prominent as the regulation mediated by the miR159–RhCKX6 module. However, we cannot rule out the possibility that miR319 and TCPs contribute to petal growth regulation in roses.
The miR159–RhCKX6 module regulates the duration of the cell division phase through modulating cytokinin catabolism in petals
To investigate the role of RhCKX6 in petal growth, we silenced RhCKX6 using a virus-induced gene silencing (VIGS) approach (Fig. 4a, b). We designed the TRV-CKX6 construct using a gene-specific region of RhCKX6 (Supplementary Fig. 9a). To test the specific silencing of RhCKX6, we performed RT-qPCR analysis: whereas RhCKX6 levels significantly decreased relative to the TRV controls, the expression levels of RhCKX1 and RhCKX5, which have high sequence similarity to RhCKX6, were not significantly different between the RhCKX6-silenced lines and the TRV control (Supplementary Fig. 9b).
a Flower opening progression of TRV and TRV-RhCKX6 plants. The experiments were performed independently twice with similar results, and one representative result is shown. Scale bars, 2 cm. b RT-qPCR analysis of RhCKX6 transcript levels in sepals 5 days after stage 0. RhUBI2 was used as an internal control. Data are shown as means ± SD (n = 4). c Flower development periods of TRV and TRV-RhCKX6 plants. Data are shown as means ± SD (n = 10). d Size of petals in TRV and TRV-RhCKX6 plants at the fully opened stage. The outermost layer of fully expanded petals was measured. Data are shown as means ± SD n = 3). Scale bars, 1 cm. e, f Number of adaxial epidermal (e) and abaxial epidermal (f) cells in TRV and TRV-RhCKX6 petals from 1 day after floral stage 0 to the fully opened stage. Data are shown as means ± SD (n = 3). Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05; **P < 0.01; ns no significant difference).
Compared to the TRV control, flower opening progression was delayed in RhCKX6-silenced lines due to a significantly extended period between stages 0 and 2 (S0–S2; 6.20 ± 0.98 days in RhCKX6-silenced plants and 4.70 ± 0.49 days in TRV) (Fig. 4c). In contrast to the miR159-knockdown lines, the final size of RhCKX6-silenced petals was larger than that of TRV controls (Fig. 4d). In agreement with this result, the numbers of adaxial and abaxial epidermal cells in RhCKX6-silenced petals were significantly higher than those of TRV controls (Fig. 4e, f). We also tested the effects of overexpressing pre-MIR159 on rose petal size, as described previously64. Overexpressing pre-MIR159 lowered RhCKX6 transcript abundance, resulted in larger petals compared to control plants, and extended the cell division phase (from S0 to S2) (Supplementary Fig. S10). Based on these results, we conclude that the enhanced petal size resulting from RhCKX6 silencing is most likely due to a prolonged cell proliferation phase.
Since CKX enzymes catalyze the irreversible degradation of cytokinins, we monitored cytokinin contents in petals of TRV control and RhCKX6-silenced lines65,66,67. The isopentenyladenine (iP) and trans-zeatin (tZ) levels in RhCKX6-silenced petals were significantly higher than those of the TRV controls, whereas the levels of cis-zeatin-riboside (cZR), trans-zeatin-riboside (tZR), and other cytokinin metabolites did not differ significantly (Fig. 5a–d; Supplementary Data 3). We monitored iP and tZ contents in petals of STTM159 lines. Knocking down miR159 abundance was associated with significantly higher RhCKX6 transcript levels and lower iP and tZ contents relative to the TRV controls (Fig. 5e–g).
a–d Cytokinin contents in petals of TRV and RhCKX6-silenced plants. Petals were sampled 5 days after stage 0. Data are shown as means ± SD (n = 3). e RT-qPCR analysis of RhCKX6 transcript levels in petals of TRV and TRV-STTM159 plants. RhUBI2 was used as an internal control. Data are shown as means ± SD (n = 3). f, g Cytokinin contents in petals of TRV and TRV-STTM159 plants. Petals were sampled 3 days after stage 0. Data are shown as means ± SD (n = 3). Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05; **P < 0.01; ***P < 0.001; ns no significant difference).
Next, we determined the levels of iP and tZ in petals during early flower development (1 and 5 days after stage 0). The iP and tZ concentrations dropped significantly as petals grew, and reached very low levels 5 days after stage 0, which corresponds to the onset of cell expansion (Fig. 6a). We also tested whether an exogenous application of cytokinin (6-benzyladenine, 6-BA) effects petal growth and cell division duration. Indeed, 6-BA treatment significantly extended the S0–S2 period (7.20 ± 0.98 days for the 6-BA treatment and 4.90 ± 0.74 days for the mock treatment) and increased the final petal size (Fig. 6b–d). The number of adaxial and abaxial epidermal cells was significantly higher in 6-BA-treated petals than in mock-treated petals, thus mimicking the phenotypes observed in RhCKX6-silenced lines (Fig. 4).
a Cytokinin contents in petals during the early development phase. The petals were sampled at 1 and 5 days after stage 0. Data are shown as means ± SD (n = 3). Different lowercase letters above each bar indicate significant differences according to one-way ANOVA with Tukey’s multiple comparisons test (P < 0.05). b, c Flower opening progression of mock-treated and 6-BA-treated rose plants. For (c), Scale bars, 2 cm. Data are shown as means ± SD (n = 10). d Petal size of mock-treated and 6-BA-treated rose at the fully opened stage. The outmost layer of fully expanded petals was measured. Data are shown as means ± SD (n = 3). Scale bars, 1 cm. e, f Number of adaxial epidermal (e) and abaxial epidermal (f) cells in mock-treated and 6-BA-treated petals from 1 day after floral stage 0 to the fully opened stage. Data are shown as means ± SD (n = 3). Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05; **P < 0.01; ***P < 0.001; ns no significant difference).
These results indicate that the miR159–RhCKX6 module governs the progression of cell division by controlling the clearance of active cytokinins in petals during early petal development.
A MYB–TPL–HDA19 complex regulates MIR159 expression in petals by modulating H3K9ac levels at the MIR159 promoter
To explore the regulatory mechanism of MIR159 expression in rose petals, we isolated a 1500-bp MIR159 promoter fragment. Promoter sequence analysis identified multiple binding sites for MYB transcription factors (Supplementary Fig. 11a). We generated MIR159 promoter fragments (P1–P3) and tested their transactivation potential in rose when driving the expression of the β-GLUCURONIDASE (GUS) reporter gene. The P2 fragment (352–713 bp upstream from the transcription start site) exhibited the strongest transcriptional activity when transiently infiltrated in rose petals (Supplementary Fig. 11b). We therefore carried out a yeast one-hybrid (Y1H) assay using the P2 fragment to screen a cDNA library prepared from RNA extracted from mixed S0 and S2 rose petals. We obtained 39 candidate clones, including several encoding transcription factors (Supplementary Data 4). Further Y1H assays confirmed that only RchiOBHmChr2g0106671, a putative MYB transcription factor, binds to the P2 region of the MIR159 promoter (Fig. 7a, Supplementary Fig. 11c). As RchiOBHmChr2g0106671 is closely related to Arabidopsis MYB73 (At4g37260) based on a phylogenetic analysis, we named this protein RhMYB73 (Supplementary Fig. 12a). A search for conserved domains identified an R2R3 MYB domain in the N terminus and an ERF-associated amphiphilic repression (EAR) motif (LSLSL) in the C terminus (Supplementary Fig. 12b, c). An electrophoretic mobility shift assay (EMSA) confirmed the direct binding of RhMYB73 to the MIR159 promoter in vitro (Fig. 7b). Subcellular localization suggested that RhMYB73 localizes to the nucleus (Fig. 7c). We also determined that RhMYB73 expression significantly increases from the S0 to S2 stages by RT-qPCR (Fig. 7d), which was opposite to the MIR159 expression pattern. Transcriptional activity, as revealed by dual-luciferase activity assays, demonstrated that RhMYB73 is a transcriptional repressor (Fig. 7e). Indeed, co-infiltrating RhMYB73 with the firefly luciferase gene (LUC) driven by the MIR159 promoter resulted in lower LUC activity in planta compared to the empty effector construct (Fig. 7f). Taken together, these results demonstrate that RhMYB73 directly binds to the MIR159 promoter and represses its expression.
a Yeast one-hybrid (Y1H) analysis of RhMYB73 binding to the MIR159 promoter. “+” and “−” indicate synthetic defined (SD) medium lacking Leu with (+) or without (–) 150 ng mL–1 Aureobasidin A (AbA). 1×, 10×, and 100× indicate the dilution factor of the yeast cultures before spotting onto the indicated medium (1, 10, and 100 times). b Electrophoretic mobility shift assay (EMSA) showing that RhMYB73 directly binds to the MIR159 promoter. The region from –392 to –432 bp of the MIR159 promoter was used to generate a probe. One microgram of purified recombinant GST-RhMYB73 was incubated with 2 nM biotin-labeled MIR159 promoter probe. As indicated, RhMYB73-dependent mobility shifts were detected and competed by an unlabeled cold probe in a dose-dependent manner. c Subcellular localization of RhMYB73 in N. benthamiana leaves. RhMYB73-GFP was co-infiltrated with NF-YA4-mCherry (nuclear marker) into N. benthamiana leaves. Fluorescence signal was visualized by confocal microscopy 3 days after infiltration. Scale bars, 50 μm. d RT-qPCR analysis of RhMYB73 transcript levels in rose petals 1, 3, and 5 days after stage 0. RhUBI2 was used as an internal control. Data are shown as means ± SD (n = 3). e Transcriptional repressor activity of RhMYB73 in N. benthamiana leaves. Left, diagrams of reporter and effector constructs. Middle images, brightfield and LUC activity. Right, quantitative analysis. Data are shown as means ± SD (n = 5). f Repression of transactivation of the MIR159 promoter by RhMYB73. Left, diagrams of reporter and effector constructs. Middle images, brightfield and LUC activity. Right, quantitative analysis. Data are shown as means ± SD (n = 3). The experiments were performed independently twice with similar results, and one representative result is shown. Different lowercase letters (in (d) and (e)) indicate significant differences according to one-way ANOVA with Tukey’s multiple comparisons test (P < 0.05). Asterisks (in (f)) indicate statistically significant differences (two-sided Student’s t-test; **P < 0.01).
To further characterize the role of RhMYB73 in petal development, we silenced RhMYB73 using VIGS. We chose a gene-specific region of RhMYB73 to generate the TRV-RhMYB73 construct (Supplementary Fig. 12d). RT-qPCR analysis showed that RhMYB73 expression decreased (Fig. 8c), whereas that of RhMYB70 and RhMYB77, which share high sequence similarity with RhMYB73, was not altered in RhMYB73-silenced lines, indicating that RhMYB73 was specifically silenced (Supplementary Fig. 12e).
a, b Flower opening progression for TRV and TRV-RhMYB73 plants. Data are shown as means ± SD (n = 10). Scale bars, 2 cm. c RT-qPCR analysis of RhMYB73 transcript levels in sepals 5 days after stage 0. RhUBI2 was used as an internal control. Data are shown as means ± SD from four biological replicates (n = 4). d Size of petals in TRV and TRV-RhMYB73 plants at the fully opened stage. The outermost layer of fully expanded petals was measured. Data are shown as means ± SD (n = 3). Scale bars, 1 cm. e, f Number of adaxial epidermal (e) and abaxial epidermal (f) cells in TRV and TRV-RhMYB73 petals from 1 day after floral stage 0 to the fully opened stage. Data are shown as means ± SD from three biological replicates (n = 3). g RT-qPCR analysis of miR159 abundance and RhCKX6 transcript levels in petals of TRV and TRV-RhMYB73 plants. RhUBI2 was used as an internal control. Data are shown as means ± SD (n = 3). h Contents of tZ (left) and iP (right) in TRV and TRV-RhMYB73 petals 5 days after stage 0. Data are shown as means ± SD (n = 3). The experiments were performed independently twice with similar results, and one representative result is shown. Asterisks indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05; **P < 0.01; ns no significant difference).
Notably, the earlier developmental window (stages S0–S2) of RhMYB73-silenced lines (5.60 ± 0.92 days) was significantly longer than that of the TRV controls (4.50 ± 0.67 days) (Fig. 8a–c). In contrast to the smaller petals of STTM159 lines, the final size of RhMYB73-silenced petals was significantly larger than that of TRV controls (Fig. 8d), with more adaxial and abaxial epidermal cells (Fig. 8e, f). Compared to TRV controls, RhMYB73 silencing caused a significant increase in miR159 abundance but lower RhCKX6 transcript levels (Fig. 8g). In addition, the iP and tZ contents were also significantly higher in RhMYB73-silenced petals compared to the TRV controls (Fig. 8h). These results indicate that RhMYB73 regulates MIR159 transcription and consequently the duration of the cell division phase in petals.
RhMYB73 harbors a conserved EAR motif, which has been well documented as a transcriptional repressor domain, mostly via recruiting chromatin remodeling factors68,69,70. We thus performed an immunoprecipitation-mass spectrometry (IP-MS) assay to identify possible RhMYB73-interacting partners and obtained 106 candidate proteins (Supplementary Data 5). Among them, we confirmed the interaction between RhMYB73 and the WD40-like family member RchiOBHmChr2g0134371 by yeast two-hybrid (Y2H) assay (Fig. 9a; Supplementary Fig. 13a). Phylogenetic analysis showed that RchiOBHmChr2g0134371 is closely related to Arabidopsis TOPLESS (TPL, At1g15750) and TOPLESS-RELATED1 (TPR1, At1g80490) (Supplementary Fig. 13b); thus, we named this protein RhTPL.
a Yeast two-hybrid assay of the interaction between RhMYB73 and RhTPL. Synthetic defined (SD) medium –TL, SD –Trp –Leu; SD –TLHA + X-gal, SD –Trp –Leu –His –Ade + X-gal. Positive control, pGBKT7-53 + pGADT7-T; negative control, pGBKT7-lam + pGADT7-T. b Bimolecular fluorescence complementation assay testing the interaction between RhMYB73, RhHDA19, and RhTPL. N. benthamiana leaves were infiltrated with the construct combinations RhMYB73-cYFP + RhHDA19-nYFP, RhMYB73-nYFP + RhTPL-cYFP, and RhTPL-cYFP + RhHDA19-nYFP. The images were captured by confocal microscopy 3 days after infiltration. Scale bars, 20 μm. c Co-immunoprecipitation assay for the interaction between RhMYB73, RhHDA19, and RhTPL. Left, RhTPL and RhMYB73; right, RhTPL and RhHDA19. RhTPL-FLAG was co-infiltrated with RhMYB73-GFP or RhHDA19-GFP in N. benthamiana leaves. Total proteins were extracted 3 days after infiltration and the supernatant with soluble proteins was incubated with an anti-GFP antibody. The precipitates were analyzed by immunoblotting with anti-FLAG and anti-GFP antibodies. d, e Chromatin immunoprecipitation-quantitative PCR assay of the relative H3K9ac levels at the MIR159 promoter in petals during early petal development (d) and in RhMYB73-silenced plants (e). Upper panel in (d), diagram of the RhMYB73-binding site in the MIR159 promoter. For (d), the petals were sampled 1, 3, and 5 days after stage 0. Data are shown as means ± SD (n = 3). f Co-infiltration of RhHDA19 and/or RhTPL strengthens RhMYB73-mediated transcriptional suppression of MIR159 in N. benthamiana leaves. Brightfield image (upper left), luciferase activity image (upper right), and quantitative analysis (bottom) of the transcriptional inhibition of the MIR159 promoter by infiltration with the effector constructs RhMYB73, RhMYB73 + RhHDA19, RhMYB73 + RhTPL, and RhMYB73 + RhHDA19 + RhTPL. Data are shown as means ± SD (n = 7). The experiment was repeated independently three times, and one representative result is shown. Different lowercase letters (in (d) and (f)) indicate significant differences according to one-way ANOVA with Tukey’s multiple comparisons test (P < 0.05). Asterisks (in (e)) indicate statistically significant differences (two-sided Student’s t-test; *P < 0.05; **P < 0.01; ns no significant difference).
Mutating the EAR motif in RhMYB73 abolished its interaction with RhTPL, indicating that the EAR motif is indispensable for this interaction (Fig. 9a). The EAR motif acts as a scaffold for the TPL–Histone deacetylase (HDAC)–Polycomb repressive complex2 (PRC2) complex during petal organogenesis71,72. Therefore, we characterized the expression levels of HDAC genes during early petal development (stages 0–2). Of the 10 HDAC genes tested, HDA6 (RchiBHmChr7g0192801) and HDA19 (RchiBHmChr6g0281731) expression substantially increased in petals during the transition from cell proliferation (stage 0) to cell expansion (stage 2) (Supplementary Fig. 13c, d). We conducted a bimolecular fluorescence complementation (BiFC) assay to test the interaction of RhTPL with RhHDA6 or RhHDA19 in N. benthamiana leaves co-infiltrated with the appropriate constructs and determined that RhTPL interacts with RhHDA19, but not with RhHDA6 (Fig. 9b; Supplementary Fig. 13e). Furthermore, BiFC assays showed that RhMYB73, RhTPL, and RhHDA19 interact with each other in nuclei in planta (Fig. 9b). A co-immunoprecipitation (co-IP) assay confirmed the RhMYB73–RhTPL and RhTPL–RhHDA19 interactions (Fig. 9c). RT-qPCR analysis indicated that RhTPL and RhHDA19 expression increases significantly in petals during the progression from cell proliferation (stage 0) to cell expansion (stage 2) (Supplementary Fig. 13f, g).
HDA19 acetylates histone H3 on lysine 9 (K9) and K14 in plants73. Therefore, we monitored H3K9ac and H3K14ac levels at the MIR159 promoter during early petal development (stages 0–2) by chromatin immunoprecipitation (ChIP)-qPCR. H3K9ac levels were significantly lower in fragments of the MIR159 promoter recognized by RhMYB73 (P5 and P6) as petals progressed from S0 to S2 (Fig. 9d), whereas H3K14ac levels remained constant levels during the same developmental window (Supplementary Fig. 13h). Therefore, we monitored H3K9ac levels of the MIR159 promoter in TRV control and RhMYB73-silenced lines. As shown in Fig. 9e, compared to the TRV control, H3K9ac levels were significantly elevated at the P5 and P6 sites of the MIR159 promoter in RhMYB73-silenced lines. Finally, a transactivation assay demonstrated that the presence of RhTPL and HDA19 significantly strengthens the repression of MIR159 transcription imposed by RhMYB73 (Fig. 9f).
Taken together, we demonstrated that the MYB73–TPL–HDA19 repression complex inhibits MIR159 transcription by modulating H3K9ac at its promoter. The resulting decrease in miR159 levels leads to CKX6 transcript, and presumably CKX6 enzyme, accumulation, and thus precocious cytokinin clearance, consequently initiating an earlier transition to cell expansion in petals. This work describes a mechanism regulating the transition from cell proliferation to expansion that controls lateral organ size (Supplementary Fig. 14).
Discussion
Cytokinin accumulation is vital to lateral organ development through controlling the duration of the cell division phase
The final size of a given organ or tissue is dictated by the number of cells that comprise it and their size, which requires coordination between cell proliferation and expansion. The transition from cell proliferation to expansion is spatially and temporally regulated by a complex network that involves multiple phytohormones. Here, we discovered that a decrease in iP and tZ levels is associated with the onset of cell expansion in rose petals. It is important to note that lower cytokinin levels were achieved by triggering cytokinin catabolism through the accumulation of a cytokinin oxidase/dehydrogenase, RhCKX6. In agreement, exogenous application of cytokinin extended the duration of the cell division phase and delayed expansion-driven flower opening, suggesting that excess cytokinin impedes normal petal growth.
An earlier study integrating transcriptome profiling and image analysis showed that cytokinin-activating genes are rapidly downregulated during the transition from proliferation to expansion in leaves, suggesting that cytokinins are involved in cell cycle arrest10. Moreover, cytokinins control the transition from the G1/S to G2/M phase during cell cycle progression by promoting the expression of CycD3 and Cyclin-dependent kinases74,75,76. Consistent with this notion, a recent study showed that cytokinin levels peaked during vein and stomatal formation and then dropped sharply during cell expansion in leaves18.
Cytokinin excess or deficiency also leads to abnormal leaf growth and smaller leaves18,77. Therefore, we propose that cytokinin homeostasis is vital for regulating the duration of the cell division phase. The temporally accurate clearance of cytokinins and the dampening of cytokinin responses are required for the normal development and growth of lateral organs and tissues, such as leaves and petals.
miR159 regulates cytokinin catabolism by targeting RhCKX6 transcripts
miR159 is an ancient and evolutionarily conserved miRNA that is present in all tested eudicot and monocot plants, as well as most angiosperms, gymnosperms, ferns, and lycopods41. The most conserved targets of miR159 encode GAMYB or GAMYB-like family proteins42,43,44,45,46. The miR159–GAMYB module influences multiple aspects of plant development41,44. In Arabidopsis, miR159 targets MYB33 and MYB65 to maintain normal leaf and flower development. Inhibiting miR159 function or mutating the miR159-binding site in MYB33 and MYB65 transcripts leads to developmental defects, such as stunted plants and curled leaves46,78. Conversely, overexpressing MIR159 results in early flowering in Arabidopsis, rice (Oryza sativa), and wheat (Triticum aestivum) due to the downregulation of GAMYB33 and GAMYB65 transcript levels79,80,81. Moreover, the miR159–MYB33 module is involved in juvenile phase control since MYB33 directly binds to the MIR156 promoter and that of its other target gene SQUAMOSA PROMOTER BINDING PROTEIN-LIKE9 (SPL9)82. In tomato, the miR159–GAMYB module acts in ovules, and overexpressing MIR159 leads to precocious fruit initiation and seedless fruits83.
Besides these conserved GAMYB targets, about 20 other transcripts have been identified as miR159 targets in Arabidopsis according to bioinformatics analyses, several of which have been experimentally validated, including 1-AMINOCYCLOPROPANE-1-CARBOXYLATE SYNTHASE8 (ACS8, At4g37770) and miRNA-REGULATED GENE1 (MRG1, At2g34010)50,51.
In this study, we discovered that RhCKX6 is a non-canonical target of miR159. We identified a miR159-binding site in the third exon of RhCKX6 and detected authentic cleavage of RhCKX6 transcripts in rose petals. Reduced miR159 abundance led to RhCKX6 transcript accumulation and cytokinin catabolism, which consequently stopped cell division. Notably, RhCKX6 was recently reported to regulate petal dehydration tolerance and senescence, similar to a gene downstream of the NAC transcription factor RhNAP, suggesting that RhCKX6 plays multiple roles in petal growth and in the response to abiotic stress84.
Notably, the expression of two predicted canonical GAMYB targets of miR159, RhMYB65 and RhMYB101, was barely detectable in rose petals during petal growth. The expression of RhMYB33, another GAMYB, remained relatively constant and declined slightly in fully opened petals. Unlike cytokinins, GA levels did not exhibit significant changes in petals. GA3 and GA7 levels were hardly detectable, whereas GA1 and GA19 levels remained stable throughout the tested developmental timeline in the petals. It appears that GA-responsive GAMYBs do not contribute to petal cell division in rose.
miR159 and miR319 exhibit high sequence similarity and share overlapping target sites63. In Arabidopsis, miR319 targets transcription factor genes from the TCP family to control floral organ morphology. Expressing a miR319-resistant form of TCP4 under the control of the petal- and stamen-specific APETALA3 (AP3) promoter led to a complete absence of petals and stamens40, indicating that the miR319–TCP4 module is involved in petal and stamen primordia initiation. In rose petals, small RNA blot and RT-qPCR analyses demonstrated that miR319 abundance is much lower than that of miR159 and remains relatively constant during petal growth. Furthermore, the expression levels of three predicted miR319 targets (TCP2, TCP4, and TCP4-X1) did not change in miR159-knockdown lines. We thus propose that the miR319–TCPs module plays a modest role in regulating petal growth compared to the miR159–RhCKX6 module in roses. However, it is still possible that the miR319–TCPs module contributes to petal growth regulation and further investigation is required to verify its involvement.
In conclusion, the miR159–RhCKX6 module represents a regulatory mechanism controlling the duration of the cell division phase in petal growth.
MIR159 expression is regulated by an epigenetic suppression complex
Here, we determined that MIR159 expression is developmentally regulated by a repressive epigenetic complex. The R2R3 MYB transcription factor RhMYB73 directly bound to the MIR159 promoter. RhMYB73 harbors an EAR motif and recruited RhTPL, which in turn interacted with RhHDA19. This MYB73–TPL–HDA19 complex modulated H3K9ac levels at the MIR159 promoter to determine the transcriptional output of MIR159, consequently influencing cytokinin catabolism in petals.
In Arabidopsis leaves, cytokinin responses are also controlled by a chromatin remodeling complex. The CIN-TCP transcription factor TCP4 interacts with the Switch/Sucrose Non-Fermentable (SWI/SNF) ATPase BRAHMA (BRM) and thus activates ARABIDOPSIS RESPONSE REGULATOR16 (ARR16) and ARR6 expression by modifying the chromatin state of their promoters. As negative regulators of the cytokinin response, higher levels of ARR16 and ARR6 reduce leaf sensitivity to cytokinins and promote cell expansion and leaf maturation77. Therefore, cytokinin homeostasis (in this study) and response are tightly controlled by epigenetic mechanisms during the transition from cell proliferation to cell expansion, indicating that cytokinin levels are crucial for proper lateral organ development.
Methods
Plant materials and growth conditions
Rose (Rosa hybrida cv. ‘Samantha’) plantlets were harvested from commercial greenhouses for propagation85. Explants at the one-node-stem were cultured on propagation medium for 30 days and then transferred to rooting medium for 30 days86. The rooted plants were transplanted into pots (9-cm diameter) containing peat soil:vermiculite (1:1, v/v) and grown at 22 ± 1 °C, with 50% relative humidity, under a 16-h-light/8-h-dark photoperiod and 100 μmol m–2 s–1 illumination with fluorescent lamps (SINOL, SN-T5, and 16 W). Nicotiana benthamiana plants were grown in the same conditions.
Virus-induced gene silencing (VIGS)
VIGS was performed as described in Zhang et al.85. Gene-specific fragments for RhMYB73 (402 bp), STTM159 (short tandem target mimic of miR159, 96 bp), and RhCKX6 (408 bp) containing EcoRI and KpnI restriction sites at either end were used to construct the plasmids pTRV2-RhMYB73, pTRV2-STTM159, and pTRV2-RhCKX6, respectively. Agrobacterium (Agrobacterium tumefaciens) strain GV3101 individually carrying pTRV2-RhMYB73, pTRV2-STTM159, or pTRV2-RhCKX6 was grown in LB medium containing 50 mg L–1 kanamycin and 50 mg L–1 rifampicin and shaken at 200 rpm at 28 °C for 16 h. Agrobacterium cells were collected by centrifugation at 5000 rpm for 8 min at room temperature and resuspended in infiltration buffer (10 mM MgCl2, 200 mM acetosyringone, and 10 mM 2-(N-Morpholino)-ethanesulfonic acid, pH 5.6) to a final OD600 = 1.5. The cultures of pTRV1 and pTRV2-RhMYB73, pTRV2-STTM159, and pTRV2-RhCKX6 were mixed in a 1:1 (v/v) ratio and incubated in the dark at room temperature for 3–4 h. Entire rose plants were immersed in infiltration buffer and exposed to –25 kPa vacuum for 10 min, twice. The infiltrated plants were briefly washed with deionized water and transplanted to pots containing peat soil:vermiculite (1:1, v/v) and grown at 22 ± 1°C, with 50% relative humidity, under a 16-h-light/8-h-dark photoperiod. Before analysis, RhMYB73-silenced, STTM159 knockdown, and RhCKX6-silenced plants were identified by determining the transcript levels of the respective gene (RhMYB73, STTM159, or RhCKX6) by RT-qPCR in sepals. According to our previous research, phenotypes of early flower development were monitored and analyzed when floral buds reached stage 0 (bud revealed)56,59,60,87. The number of plants displaying the selected phenotypes for each VIGS line TRV2-STTM159, RhCKX6, and RhMYB73 were 61/116, 53/126, and 57/122, respectively. The primers used in the VIGS assay are listed in Supplementary Data 6.
5′ RNA ligase-mediated rapid amplification of cDNA ends (5′ RLM-RACE)
The 5′ RLM-RACE assay was performed according to the manual of the FirstChoice RLM-RACE kit (No. AM1700). Total RNA was extracted with a pBIOZOL kit (BioFlux, BSC55Ml) from rose and wild strawberry petals, treated with calf intestine alkaline phosphatase (CIP) and tobacco acid pyrophosphatase, and ligated with RNA adapters at 37 °C for 1 h. Reverse transcription and nested PCR for 5′ RLM-RACE (outer and inner) PCR were performed according to the manufacturer’s instructions. The second PCR products were ligated into the pEASY-Blunt Cloning Vector (No. CB111; Transgene, Beijing, China) for sequencing. Primers used in RLM-RACE are listed in Supplementary Data 6.
Quantification of endogenous cytokinins
About 300 mg (fresh weight) of rose petals were harvested, immediately frozen in liquid nitrogen, and stored at −70 °C. The cytokinin contents were detected by MetWare (Wuhan, China) based on the AB Sciex QTRAP 6500 LC-MS/MS platform (UPLC, ExionLC AD, https://sciex.com.cn/; MS, Applied Biosystems 6500; Triple Quadrupole, https://sciex.com.cn/). Three biological replicates were performed for each sample.
Exogenous 6-benzylaminopurine (6-BA) treatment
The 6-BA treatment was performed following the procedure described previously88. Flower buds (bud revealed but ahead of stage 0) were sprayed with 100 μM 6-BA every day until 5 days after stage 0.
Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR)
The ChIP-qPCR was performed as described in Chen et al.87. Briefly, 1.5 g of rose petals (collected at 1, 3, and 5 days after floral buds reached stage 0) was crosslinked in 1% (w/v) polyformaldehyde. Chromatin was fragmented into >500-bp segments via sonication, followed by incubation and immunoprecipitation with a specific antibody against acetylated histone H3K9/K14 (H3K9ac, 1:5000 dilution, Abcam, No. ab10812; H3K14ac, 1:5000 dilution, Millipore, No. 07-353). The immunoprecipitated DNA fragments were analyzed by quantitative PCR. The primers used in ChIP-qPCR are listed in Supplementary Data 6.
Small RNA blot analysis of miRNA
Total RNA was extracted from rose petals with CTAB reagent. Small RNA blotting was performed following the protocol described in Lee et al. and Kim et al.89,90. Briefly, 20 μg RNA was separated on a 15% urea-polyacrylamide gel and transferred to a nylon membrane (Millipore, No. INYC00010) using a semidry transfer cell (Bio-Rad, Parameter: 390 mA, 20–30 min). Subsequently, hybridization was performed according to a standard protocol. Locked Nucleic Acids (LNA)-modified oligonucleotide probes were used to ensure the specificity of detection for miR159 and miR31991. The probes corresponding to miR159 and miR319 were 5′-taGAGCTCCCTTCAATCCAAa-3′ and 5′-ggAGCTCCCTTCAGTCCAAt-3′, respectively (lowercase letters indicate the LNA-modified oligonucleotides). U6 (5′-AGGGGCCATGCTAATCTTCTC-3′) was used as an internal control.
Stem-loop RT-qPCR for miRNA
Total RNA was extracted from rose petals with CTAB reagent. Reverse transcription was performed with 200 ng total RNA with a miRNA-specific stem-loop RT primer using Takara PrimeScript RT reagent kit (RR037A). qPCR was performed according to a previously described method using a miRNA-specific forward primer and a universal reverse primer92. 5S rRNA was used as an internal control.
Microscopy observation and cell counting
Cell imaging and cell counting were performed as described by Ma et al. and Yamada et al.55,56. The outermost petals were peeled off and fixed by formaldehyde-acetic acid (FAA) solution (3.7% formaldehyde, 5% glacial acetic acid, 50% ethanol) and decolorized with an ethanol gradient series. The adaxial and abaxial epidermal cells of the petals were observed with an optical microscope and photographed. The number of cells was counted using Image J (National Institutes of Health, USA) per visual field. The total number of adaxial and abaxial epidermal cells per petal was calculated by dividing the total petal area by the number of cells per unit area.
RNA extraction and quantitative RT-PCR (RT-qPCR)
Total RNA was extracted from rose petals by a pBIOZOL kit (BioFlux, BSC55Ml)88. Genomic DNA contaminants were removed by digestion with RNase-free DNase I (Promega). Reverse transcription was performed with 1 μg total RNA using HiScript II Q Select RT SuperMix kit (Vazyme, Nanjing, China). qPCR was performed on a StepOne Real-Time PCR System (Applied Biosystems, Carlsbad, USA) and a KAPA SYBR FAST qPCR kit (Kapa Biosystems, Wilmington, MA, USA). RhUBI2 was used as an internal control86.
RNA-seq
Total RNA was extracted from rose petals as described in Wu et al. and Tian et al.88,93, and genomic DNA contamination was removed using RNase-free DNase I (Promega). The quality and quantity of the RNA were assessed using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). Samples with an RNA integrity number (RIN) > 8 were used for RNA-seq by Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). One microgram of total RNA per sample was used for cDNA library construction and Illumina sequencing, and RNA-seq data were processed, assembled, and annotated as previously described94,95. Clean RNA-seq reads were aligned to the reference genome (Rosa chinensis cv. Old Blush, GenBank ID 8255808) and deposited in the NCBI BioProject database under the accession number PRJNA808873. Finally, differentially expressed genes (DEGs) were analyzed according to the parameters (fold change ≥ 2 or ≤0.5, q-value ≤ 0.05) and subjected to GO and KEGG enrichment analyses. The stage 0 and stage 2 microarray datasets were obtained from the rose transcriptome database (http://bioinfo.bti.cornell.edu/rose).
Subcellular localization
The coding sequence of RhMYB73 was cloned in-frame of the GFP sequence into the pSuper vector containing Xma I and Kpn I restriction sites. pSuper:NF-YA4-mCherry was used as a nuclear marker85. The constructed vectors were transformed into Agrobacterium strain GV3101 and co-infiltrated into N. benthamiana leaves for 3 days. Subcellular localization was monitored in leaf epidermal cells by confocal laser scanning microscopy (Olympus, FV3000, Japan) at 488 nm and 561 nm excitation, respectively. The primers used in the assay are listed in Supplementary Data 6.
GUS staining and yeast one-hybrid assay (Y1H)
A 1500-bp fragment of the MIR159 promoter was cloned based on the rose genome sequence (https://lipm-browsers.toulouse.inra.fr/pub/RchiOBHm-V2/) with primers using genomic DNA from the cultivar ‘Samantha’ as template. The cloned MIR159 promoter (–1000 to –1 bp relative to the transcription start site of MIR159) was truncated into three short segments (P1, P2, and P3) and then cloned into a modified 35 Smini:GUS vector with a minimal 35S promoter upstream of the GUS reporter gene. GUS staining was performed 3 days after infiltration of rose petals through Agrobacterium-mediated assays. After analysis of MIR159 promoter activity, the MIR159 promoter fragment with highest GUS activity and flanked by Hind III and Sal I restriction sites was inserted into the pAbAi vector to generate the reporter construct pAbAi-proMIR159-P2. In parallel, the coding sequence of RhMYB73 flanked by EcoR I and Xho I restriction sites was inserted into the pGADT7 vector to generate the effector construct pGADT7-RhMYB73. The Y1H assay was performed to examine the interaction between RhMYB73 and the MIR159 promoter according to manual provided by Matchmaker Gold Y1H Library Screening System (Clontech, Dalian, China). The resulting vectors were transformed into yeast strain Y1H gold, and the transformants were grown for 3 days on synthetic defined (SD) medium lacking Ura and Leu (SD –Ura –Leu) with or without 150 ng mL−1 Aureobasidin A (AbA). Primers used for Y1H are listed in Supplementary Data 6.
Electrophoretic mobility shift assay (EMSA)
The EMSA was performed as described in Pei et al. and Cheng et al.60,96. The RhMYB73 coding sequence was inserted into the pGEX-4T-2 vector at the EcoR I and Xho I restriction sites to generate pGEX-4T-RhMYB73. The resulting vector was transformed into Escherichia coli strain BL21 to produce recombinant GST-RhMYB73 fusion protein, which was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG, 0.2 mM) at 28 °C for 6 h. The GST-RhMYB73 protein was purified with glutathione Sepharose 4B (GE Healthcare, Pittsburgh, USA) following the manufacturer’s instructions. The MIR159 promoter probe was synthesized and labeled with biotin. Unlabeled and mutated biotin probes for the MIR159 promoter were used as competitors. EMSAs were performed using a chemiluminescent nucleic acid detection module kit (Thermo Fisher, Waltham, USA). The primers used in EMSA are listed in Supplementary Data 6.
Dual-LUC reporter assay
The dual-LUC reporter assay was performed as described in Zhang et al.86. For transcriptional activity analysis, the coding sequence of RhMYB73 was inserted into the pBD vector97. The transcriptional activity of RhMYB73 was compared to the positive (pBD-VP16) and negative (pBD) controls98. For the transcriptional inhibition of MIR159 transcription by RhMYB73, the coding sequence of RhMYB73 was inserted into the pGreenII 62-SK vector to construct the 35S:RhMYB73 effector plasmid, and the MIR159 promoter with 1000 bp in length was inserted into pGreenII 0800-LUC to construct the MIR159pro:LUC reporter plasmid. The resulting vectors were individually transformed into Agrobacterium strain GV3101 (harboring the pSoup plasmid) and co-infiltrated into N. benthamiana leaves. Three days later, we cut the tobacco leaves that have been injected with Agrobacterium and sprayed a reaction solution containing 50 mg L−1 of luminol substrate D-luciferin onto the abaxial surface of the leaves. The reaction was carried out for 5 min in the dark, and then luciferase activity images were captured with a CCD camera (CHEMIPROHT 1300B/LND, 16 bit; Roper Scientific, Sarasota, FL, USA) to detect luminescence, with an exposure frequency of 100 KHz for 10 min. The ratios between firefly (LUC) and Renilla (REN) luciferase activities were measured using dual-LUC assay reagents (Promega, Madison, WI, USA). Primers used for the LUC assay are listed in Supplementary Data 6.
Phylogenetic analysis
Alignment was performed with ClustalW (https://www.genome.jp/tools-bin/clustalw). The phylogenetic tree was constructed based on the alignment results using the maximum likelihood algorithm in MEGA version X software with 1000 bootstrap replicates.
Yeast two-hybrid assay (Y2H)
The Y2H was performed as described in Chen et al.87. The coding sequences of RhMYB73 and RhMYB73-mEAR (with a mutated EAR sequence) were inserted into pGBKT7 (BD) to construct RhMYB73-BD as bait vector. The coding sequence of RhTPL was inserted into pGADT7 (AD) to construct RhTPL-AD as prey vector. The bait and prey vectors, positive control vectors (pGBKT7-53 and pGADT7-T), and negative control vectors (pGBKT7-lam and pGADT7-T) were transformed into yeast strain Y2H Gold. The positive colonies were transferred to different growth media (SD –TL, SD –Trp –Leu; SD –TLHA, SD –Trp–Leu–His–Ade; SD –TLHA X-gal SD –Trp–Leu–His–Ade + X-gal). The primers used in Y2H are listed in Supplementary Data 6.
Bimolecular fluorescence complementation (BiFC)
The BiFC was performed as described in Chen et al.87. The coding sequences of RhMYB73, RhHDA19, and RhTPL were cloned in-frame with the sequence encoding the N-terminal half of YFP (nYFP) or the C-terminal half of YFP (cYFP) to generate RhMYB73-nYFP, RhMYB73-cYFP, RhHDA19-nYFP, and RhTPL-cYFP vectors. The appropriate pairs of constructs (RhMYB73-nYFP and RhTPL-cYFP; RhMYB73-cYFP and RhHDA19-nYFP; RhHDA19-nYFP and RhTPL-cYFP) were infiltrated into N. benthamiana leaves via Agrobacterium-mediated transient infiltration. At 3 days after infiltration, YFP and mCherry fluorescence was detected by confocal microscopy (Olympus, FV3000, Japan) with excitation at 488 and 561 nm, respectively.
Immunoprecipitation-mass spectrometry (IP-MS)
For IP-MS assay, total proteins were extracted using a Total Protein Extraction Kit for Plant Tissues (Minute™, inventbiotech, Beijing, China) and incubated with recombinant GST or GST-RhMYB73 for 2 h at 4 °C. Supernatant was transferred to a fresh tube and purified with glutathione Sepharose 4B (GE Healthcare, Pittsburgh, USA) following the manufacturer’s instructions. The samples were collected with centrifugation at 5000 rpm at 4 °C for 10 min. The purified proteins were fractionated on 10% (v/v) SDS-PAGE gels and digested with trypsin overnight incubation at 37 °C for subsequent MS analyses. The resulting MS/MS data were processed using the MaxQuant search engine (v.1.5.2.8)99. The instrument was equipped with an electrospray ionization-trap (ESI-TRAP). The search parameters were as follows: mass tolerance was set to 20 parts per million (ppm) for the search. The fragment mass tolerance was ±0.02 Da. Trp-C/M was specified as the cleavage enzyme while allowing up to two missing cleavages. The raw mass spectrometry data of validation have been deposited to the ProteomeXchange Consortium under accession number PXD046197. The primers used in IP-MS are listed in Supplementary Data 6.
Co-immunoprecipitation assays (Co-IP)
For Co-IP assays, Agrobacterium harboring 35S:RhHDA19-GFP was co-infiltrated with 35S:RhTPL-FLAG into N. benthamiana leaves, 35S:RhMYB73-GFP was co-infiltrated with 35S:RhTPL-FLAG into N. benthamiana leaves, and co-infiltration of 35S:GFP and 35S:RhTPL-FLAG was used as a negative control. After 3 days, total proteins were extracted using extraction buffer87. The supernatant was first incubated with GFP-Trap® Agarose Beads (1:200 dilution; Chromotek, No. gta) and then analyzed by immunoblot using anti-FLAG (1:2500 dilution; Abcam, No. ab1162,) and anti-GFP (1:2000 dilution; Sigma-Aldrich, No. G1544,) antibodies. The primers used in Co-IP are listed in Supplementary Data 6.
Transient overexpression in rose bud
The pSuper::pre-MIR159 construct and pSuper were transformed into Agrobacterium tumefaciens cells (strain GV3101).The rose bud (0.5 cm) was immersed in the pSuper::pre-MIR159 and pSuper bacterial suspension for transient overexpression of MIR159, followed by infiltrating under a vacuum at 0.7 MPa64. Before analysis, vector primers pSuper-F/R were used to measure DNA level insertions in petals at the flower opening stage 2 of inoculated plants by PCR. Then, we identified the expression of miR159 and RhCKX6 in the same petals of these successfully inoculated plants by RT-qPCR and selected the overexpression plants to analyze the phenotypes.
Statistical analysis
The statistical significance of the data was tested in GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, USA: http://www.graphpad.com/). All results were tested with Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available from the corresponding author upon request. The data underlying Figs. 1b–c, e–g; 2e–f; 3c; 4b–f; 5a–g; 6a–b, d–f; 7d–f; 8b–h; 9d–f and Supplementary Figs. 1a–c; 2a; 7c–d; 8a, d; 9b; 10b–d; 12e; 13f–h are provided in the Source Data file. All primers used in this study are listed in Supplementary Data 6. Information on the genes used in this study is given in Supplementary Data 7. RNA-seq data that support the findings of this study have been deposited in the NCBI Bioproject database under accession number PRJNA808873. The raw mass spectrometry data of validation have been deposited to the ProteomeXchange Consortium under accession number PXD046197. Source data are provided with this paper.
References
Gonzalez, N., Vanhaeren, H. & Inze, D. Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci. 17, 332–340 (2012).
Bogre, L., Magyar, Z. & Lopez-Juez, E. New clues to organ size control in plants. Genome Biol. 9, 226–232 (2008).
Boulan, L. & Leopold, P. What determines organ size during development and regeneration? Development 148, dev196063–dev196071 (2021).
Hepworth, J. & Lenhard, M. Regulation of plant lateral-organ growth by modulating cell number and size. Curr. Opin. Plant Biol. 17, 36–42 (2014).
Lee, B. H. et al. The Arabidopsis GRF-INTERACTING FACTOR gene family performs an overlapping function in determining organ size as well as multiple developmental properties. Plant Physiol. 151, 655–668 (2009).
Rojas, C. A. et al. Overexpression of the Arabidopsis anaphase promoting complex subunit CDC27a increases growth rate and organ size. Plant Mol. Biol. 71, 307–318 (2009).
Eloy, N. B. et al. The APC/C subunit 10 plays an essential role in cell proliferation during leaf development. Plant J. 68, 351–363 (2011).
Schommer, C., Debernardi, J. M., Bresso, E. G., Rodriguez, R. E. & Palatnik, J. F. Repression of cell proliferation by miR319-regulated TCP4. Mol. Plant. 7, 1533–1544 (2014).
Kazama, T., Ichihashi, Y., Murata, S. & Tsukaya, H. The mechanism of cell cycle arrest front progression explained by a KLUH/CYP78A5-dependent mobile growth factor in developing leaves of Arabidopsis thaliana. Plant Cell Physiol. 51, 1046–1054 (2010).
Andriankaja, M. et al. Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Dev. Cell 22, 64–78 (2012).
Mizukami, Y. & Fischer, R. L. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl Acad. Sci. USA 97, 942–947 (2000).
Sonoda, Y. et al. Regulation of leaf organ size by the Arabidopsis RPT2a 19S proteasome subunit. Plant J. 60, 68–78 (2009).
Hu, Y., Poh, H. M. & Chua, N. H. The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J. 47, 490–490 (2006).
Achard, P. et al. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr. Biol. 19, 1188–1193 (2009).
Nelissen, H. et al. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr. Biol. 22, 1266–1266 (2012).
Spartz, A. K. et al. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 70, 978–990 (2012).
Zhiponova, M. K. et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol. 197, 490–502 (2013).
Skalak, J. et al. Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. Plant J. 97, 805–824 (2019).
Donnelly, P. M., Bonetta, D., Tsukaya, H., Dengler, R. E. & Dengler, N. G. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 215, 407–419 (1999).
Efroni, I., Eshed, Y. & Lifschitz, E. Morphogenesis of simple and compound leaves: a critical review. Plant Cell 22, 1019–1032 (2010).
Hu, Y., Xie, Q. & Chua, N. H. The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15, 1951–1961 (2003).
Krizek, B. A. Making bigger plants: key regulators of final organ size. Curr. Opin. Plant Biol. 12, 17–22 (2009).
Szecsi, J. et al. BIGPETALp, a bHLH transcription factor is involved in the control of Arabidopsis petal size. Embo J. 25, 3912–3920 (2006).
Varaud, E. et al. AUXIN RESPONSE FACTOR8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. Plant Cell 23, 973–983 (2011).
Schruff, M. C. et al. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133, 251–261 (2006).
Vert, G., Walcher, C. L., Chory, J. & Nemhauser, J. L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl Acad. Sci. USA 105, 9829–9834 (2008).
Nelissen, H. et al. The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol. J. 16, 615–627 (2018).
Strable, J. & Nelissen, H. The dynamics of maize leaf development: patterned to grow while growing a pattern. Curr. Opin. Plant Biol. 63, 102038–102047 (2021).
Lee, L. Y. C. et al. STUNTED mediates the control of cell proliferation by GA in Arabidopsis. Development 139, 1568–1576 (2012).
Sakakibara & Hitoshi CYTOKININS: activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 57, 431–449 (2006).
Werner, T. & Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 12, 527–538 (2009).
Schmitz, R. Y. et al. Cytokinins: synthesis and biological activity of geometric and position isomers of Zeatin1. Plant Physiol. 50, 702–705 (1972).
Einset, J. W. Conversion of N6-isopentenyladenine to zeatin by Actinidia tissues. Biochem. Biophys. Res. Commun. 124, 470–474 (1984).
Armstrong, D. J. Cytokinin oxidase and the regulation of cytokinin degradation. Cytokinins Chem. Activity Function 125, 139–154 (1994).
Frébort, I. et al. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 62, 2431–2452 (2011).
Werner, T. et al. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532–2550 (2003).
Bartrina, I. et al. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23, 69–80 (2011).
Rogers, K. & Chen, X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 25, 2383–2399 (2013).
Chen, X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303, 2022–2025 (2004).
Nag, A. et al. miR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proc. Natl Acad. Sci. USA 106, 22534–22539 (2009).
You, C. J. et al. Conservation and divergence of small RNA pathways and microRNAs in land plants. Genome Biol. 18, 158–176 (2017).
Aya, K. et al. The Gibberellin perception system evolved to regulate a pre-existing GAMYB-mediated system during land plant evolution. Nat. Commun. 2, 544–552 (2011).
Li, Y. J., Alonso-Peral, M., Wong, G. G., Wang, M. B. & Millar, A. A. Ubiquitous miR159 repression of MYB33/65 in Arabidopsis rosettes is robust and is not perturbed by a wide range of stresses. BMC Plant Biol. 16, 179–191 (2016).
Millar, A. A., Lohe, A. & Wong, G. Biology and function of miR159 in plants. Plants 8, 255–271 (2019).
Tsuzuki, M. et al. Profiling and characterization of small RNAs in the Liverwort, Marchantia polymorpha, belonging to the first diverged land plants. Plant Cell Physiol. 57, 359–372 (2016).
Alonso-Peral, M. M. et al. The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol. 154, 757–771 (2010).
Schwab, R. et al. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 8, 517–527 (2005).
German, M. et al. Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nat. Biotechnol. 26, 941–946 (2008).
Allen, R. et al. MicroR159 regulation of most conserved targets in Arabidopsis has negligible phenotypic effects. Silence 28, 18 (2010).
Alves, L. et al. Comprehensive prediction of novel microRNA targets in Arabidopsis thaliana. Nucleic Acids Res. 37, 4010–4021 (2009).
Buxdorf, K. et al. Identification and characterization of a novel miR159 target not related to MYB in tomato. Planta. 232, 1009–1022 (2010).
Pei, H. et al. Integrative analysis of miRNA and mRNA profiles in response to ethylene in rose petals during flower opening. PLoS ONE 8, e64290 (2013).
D’ario, M. & Sablowski, R. Cell size control in plants. Annu. Rev. Genet. 53, 45–65 (2019).
Powell, A. E. & Lenhard, M. Control of organ size in plants. Curr. Biol. 22, R360–R367 (2012).
Yamada, K. et al. Cell division and expansion growth during rose petal development. J. Jpn. Soc. Hortic. Sci. 78, 356–362 (2009).
Ma, N. et al. Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol. 148, 894–907 (2008).
Sun, X. et al. Molecular understanding of postharvest flower opening and senescence. Mol. Hortic. 1, 7–18 (2021).
Langmead, B. et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Ma, N. et al. Petal senescence: a hormone view. J. Exp. Bot. 69, 719–732 (2018).
Pei, H. et al. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol. 163, 775–791 (2013).
Liu, Y., Schiff, M. & Dinesh-Kumar, S. P. Virus-induced gene silencing in tomato. Plant J. 31, 777–786 (2002).
Yan, J. et al. Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 24, 415–427 (2012).
Reichel, M. & Millar, A. A. Specificity of plant microRNA target MIMICs: cross-targeting of miR159 and miR319. Plant Physiol. 180, 45–48 (2015).
Liang, Y. et al. Auxin regulates sucrose transport to repress petal Abscission in Rose (Rosa hybrida). Plant Cell 32, 3485–3499 (2020).
Kakimoto, T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 42, 677–685 (2001).
Miyawaki, K. et al. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl Acad. Sci. USA. 103, 16598–16603 (2006).
Hwang, I., Sheen, J. & Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 63, 353–380 (2012).
Kazan, K. Negative regulation of defence and stress genes by EAR-motif-containing repressors. Trends Plant Sci. 11, 109–112 (2006).
Ikeda, M. & Ohme-Takagi, M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 50, 970–975 (2009).
Kagale, S. & Rozwadowski, K. EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6, 141–146 (2011).
Pelayo, M. A., Yamaguchi, N. & Ito, T. One factor, many systems: the floral homeotic protein AGAMOUS and its epigenetic regulatory mechanisms. Curr. Opin. Plant Biol. 61, 102009–102019 (2021).
Huang, R., Huang, T. & Irish, V. F. Do epigenetic timers control petal development? Front. Plant Sci. 12, 709360–709365 (2021).
Chen, X. S., Ding, A. B. & Zhong, X. H. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 63, 206–216 (2020).
Riou-Khamlichi, C., Huntley, R., Jacqmard, A. & Murray, J. A. H. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544 (1999).
Inze, D. & De Veylder, L. Cell cycle regulation in plant development. Annu. Rev. Genet. 40, 77–105 (2006).
Wu, W. Q., Du, K., Kang, X. Y. & Wei, H. R. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 8, 118–131 (2021).
Efroni, I. et al. Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev. Cell 24, 438–445 (2013).
Allen, R. S. et al. Genetic analysis reveals functional redundancy and the major target genes of the Arabidopsis miR159 family. Proc. Natl Acad. Sci. USA 104, 16371–16376 (2007).
Achard, P., Herr, A., Baulcombe, D. C. & Harberd, N. P. Modulation of floral development by a gibberellin-regulated microRNA. Development 131, 3357–3365 (2004).
Li, X. et al. Flowering time control in ornamental gloxinia (Sinningia speciosa) by manipulation of miR159 expression. Ann. Bot. 111, 791–799 (2013).
Tsuji, H. et al. GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J. 47, 427–444 (2006).
Guo, C. K. et al. Repression of mir156 by mir159 regulates the timing of the juvenile-to-adult transition in Arabidopsis. Plant Cell 29, 1293–1304 (2017).
Da Silva, E. M. et al. microRNA159-targeted SlGAMYB transcription factors are required for fruit set in tomato. Plant J. 92, 95–109 (2017).
Zou, J. et al. Regulation of rose petal dehydration tolerance and senescence by RhNAP transcription factor via the modulation of cytokinin catabolism. Mol. Horticulture 1, 13 (2021).
Zhang, S. et al. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat. Plants 5, 290–299 (2019).
Zhang, Y. et al. The circadian-controlled PIF8-BBX28 module regulates petal senescence in rose flowers by governing mitochondrial ROS homeostasis at night. Plant Cell 33, 2716–2735 (2021).
Chen, J. W. et al. AUXIN RESPONSE FACTOR 18-HISTONE DEACETYLASE 6 module regulates floral organ identity in rose (Rosa hybrida). Plant Physiol. 186, 1074–1087 (2021).
Wu, L. et al. An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol. 173, 853–862 (2017).
Lee, R. C. et al. An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 (2001).
Kim, S. et al. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 38, 98–104 (2010).
Válóczi, A. et al. Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 32, e175 (2004).
Erika, V.-G. Stem-loop qRT-PCR for the detection of plant microRNAs. Plant Epigenetic. 1456, 163–175 (2017).
Tian, J. et al. TRVGFP: a modified Tobacco rattle virus vector for efficient and visualizable analysis of gene function. J. Exp. Bot. 65, 311–322 (2014).
Yang, M. et al. Transcriptomic analysis of the regulation of rhizome formation in temperate and tropical lotus (Nelumbo nucifera). Sci. Rep. 5, 13059–13075 (2015).
Zhang, S. et al. RhMYB108, an R2R3-MYB transcription factor, is involved in ethylene- and JA-induced petal senescence in rose plants. Hortic. Res. 6, 131–142 (2019).
Cheng, C. X. et al. Ethylene-regulated asymmetric growth of the petal base promotes flower opening in rose (Rosa hybrida). Plant Cell 33, 1229–1251 (2021).
Sainsbury, F., Thuenemann, E. C. & Lomonossoff, G. P. pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 7, 682–693 (2009).
Han, Y. C. et al. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 171, 1070–1084 (2016).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 26, 1367–1372 (2008).
Acknowledgements
This work was supported by the National Key Research and Development Program (Grant 2020YFD1000405, J.G., N.M., X.Z.), the National Natural Science Foundation of China (Grant number 31872148 N.M. and 32202530 W.J.), the Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF-PXM2019_014207_000032, J.G., N.M., X.Z.), and the 111 Project (B17043, J.G., N.M., X.Z.). We thank Dr. Zhizhong Gong (China Agricultural University) for providing the pSuper1300 vector and Dr. Wangjin Lu (South China Agricultural University) for providing the pBD, pBD-VP16, and dual-reporter vectors. We thank Dr. Rui Xia (South China Agricultural University) for offering technical support for the miRNA analysis.
Author information
Authors and Affiliations
Contributions
N.M. and X.Z. designed the research. W.J., F.G., G.L., J.L., Y.D. and W.Y. performed the experiments. X.S., Y.L. and J.G. provided technical support and conceptual advice. W.J., X.Z. and N.M. conducted data analysis. W.J., F.G., X.Z. and N.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jing, W., Gong, F., Liu, G. et al. Petal size is controlled by the MYB73/TPL/HDA19-miR159-CKX6 module regulating cytokinin catabolism in Rosa hybrida. Nat Commun 14, 7106 (2023). https://doi.org/10.1038/s41467-023-42914-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42914-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.