Abstract
The use of commercial long-wavelength (>650 nm) laser dyes in many biophotonic applications has several important limitations, including low absorption at the standard pump wavelength (532 nm) and poor photostability. Here, we demonstrate that the use of Förster type (FRET) energy transfer can overcome these problems to enable efficient, stable near-infrared lasing in a colloidal suspension of latex nanoparticles containing a mixture of Rhodamine 6G and Nile Blue dyes. Experimental and theoretical analyses of the photophysics suggest that the dominant energy transfer mechanism is Förster type via dipole–dipole coupling, and also reveal an unexpected core/shell morphology in the dye-doped nanoparticles. FRET-assisted incoherent random lasing is also demonstrated in solid samples obtained by evaporation of colloidal suspensions.
Similar content being viewed by others
Main
Tunable dye lasers have been demonstrated to be an important tool in a wide variety of fields, from spectroscopy to isotope separation, photochemistry, material diagnosis and medicine1,2,3. In biomedical and biophotonic applications, long-wavelength (>650 nm) fluorescent dyes have some distinct advantages linked to the fact that long-wavelength light can penetrate deeper into tissue4, which facilitates their use in surgical and photodynamic therapy treatments3. Although a number of long-wavelength-emitting commercial dyes are available that have reasonable efficiency5, these dyes have two important drawbacks: low absorption at the standard pump wavelength of 532 nm and/or rather poor photostability. One approach to overcoming these drawbacks is to use mixtures of dyes in a system based on Förster resonance energy transfer (FRET). FRET is a physical phenomenon where excitation energy from an excited donor is non-radiatively transferred to a proximal ground-state acceptor6,7,8,9,10,11,12,13,14. A system is required that consists of two dyes; one efficiently absorbs the pump radiation at 532 nm and is able to transfer the excitation energy to a second, long-wavelength emitting dye. The energy transfer process in a FRET system requires good overlap between donor emission and acceptor absorption bands; it depends strongly on the distance between donor and acceptor molecules, and, as recently reported, on the conservation or not of the angular momentum in the transfer process15. One way to ensure the proximity of the dye molecules and thus favour the energy transfer process is to confine donor and acceptor molecules within nanoparticles11,16,17. We have also recently demonstrated significant improvements in the lasing efficiency and photostability of the dye Rhodamine 6G (Rh6G) by confining it in polymeric nanoparticles (latexes) homogeneously dispersed in aqueous suspensions18,19. Consequently, we thought of using dye confinement in latex nanoparticles in combination with the FRET process as a strategy to overcome simultaneously the low absorption, low laser efficiency and photostability problems of long-wavelength emitting dyes. The red emitting dye Nile Blue (NB), which is quite efficient but rather unstable under pumping at 532 nm, seemed an ideal candidate to prove this approach, as there is a good overlap between the emission band of Rh6G and its absorption band (Supplementary Fig. S1), a necessary condition for an efficient non-radiative energy transfer.
In this Article, we report, for the first time, efficient and photostable FRET-assisted laser emission in colloidal suspensions of latex nanoparticles containing mixtures of Rh6G and NB (see Methods). The mechanism of energy transfer in this system is properly assessed by analysing the photophysical properties of the donor, acceptor and mixtures of donor and acceptor encapsulated inside the nanoparticles. A thorough theoretical analysis of the FRET process allows us to infer that the inner morphology of the latex nanoparticles is of a core–shell type. Finally, unprecedented FRET-assisted incoherent random lasing is also obtained in solid samples deposited by evaporation of the colloidal suspensions.
Laser characterization
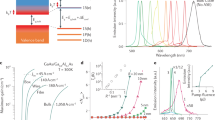
A systematic study was carried out regarding the laser performance of samples with different dye loadings and ratios and nanoparticle content in the suspension. The best compromise between laser and transfer efficiency was obtained from a colloidal suspension of 6.7 wt% of nanoparticles with a Rh6G/NB proportion of 1.5/1 (0.28/0.19 wt% with respect to monomer, respectively). The optimized laser emission (Fig. 1a) was centred at 700 nm (typical of NB), with just some residual emission from the Rh6G, and reached an efficiency (percentage of the excitation energy converted into laser emission) of 13% under transversal pumping at 532 nm. The laser output energy and spectrum suggest that the energy transfer efficiency reaches 98%, because the efficiency of a suspension containing only Rh6G is 30%, and the contribution of the Rh6G emission to the efficiency of the suspension with both Rh6G and NB is just 0.6%. (This is determined as ∫IL(λ)hc/λ dλ, integrated over the Rh6G contribution to the laser spectrum IL(λ), normalized so that ∫IL(λ)hc/λ dλ, integrated over all wavelengths, is 13%, where h is Planck's constant and c is the speed of light in vacuum.) In comparison, and indicative of this efficient energy transfer, no laser emission was obtained when the nanoparticles contained only NB at the concentration that optimized lasing in the donor/acceptor mixtures.
a, Lasing emission spectra from colloidal suspensions of poly(MMA-HEMA-GMA) nanoparticles with different relative proportions of Rh6G/NB: (from left to right) 1.5/1 (0.28/0.19 wt%), 3/1 (0.36/0.12 wt%) and 1/1 (0.09/0.09 wt%). Photographs below the spectra show the actual laser spot on a black screen placed 30 cm from the laser cavity. b, Normalized laser-induced fluorescence emission as a function of the number of pump pulses for NB in ethanol (A), a mixture of 0.024/0.016 wt% of Rh6G/NB in ethanol (B), a colloidal suspension of nanoparticles with 1.5/1 (0.28/0.19 wt%) Rh6G/NB (C).
This lack of laser emission from NB alone can be understood as being a result of a combination of two factors. First, it is well known that the composition of the material containing the dye is most important in ensuring laser emission from organic dyes20. In this regard, methyl methacrylate (MMA)/2-hydroxylethylmethacrylate (HEMA) copolymers are not the best environment for the laser action of NB, because this dye lases more efficiently in protic solvents such as ethanol (which is mimicked by the HEMA monomer) than in aprotic solvents such as ethyl acetate (which is mimicked by the MMA monomer). Thus, the presence of MMA in the nanoparticle composition does not favour laser emission from NB when this dye is alone in the nanoparticle. Second, under transversal excitation, dye concentration becomes a critical factor, because the dye samples require an optical density of ∼20 to assure that the incoming pump radiation is absorbed within a depth similar to the cross-section of that pump radiation at the input face of the cell onto which the exciting pulses are line-focused (∼0.3 mm). Then, the concentration of NB that optimizes its emission from the confined donor/acceptor mixtures is lower than that required for efficient laser emission when the NB is alone within the nanoparticle. These two factors, taken together, prevent laser emission from NB when included in nanoparticles, alone, at the concentration that is optimal for the Rh6G/NB mixtures.
The lasing efficiency and spectrum depend strongly on donor/acceptor proportion (Fig. 1). When this is increased to 2/1, lasing efficiency is slightly reduced (11%), but Rh6G residual emission increases. Higher amounts of Rh6G result in a reduced efficiency, with progressively growing Rh6G emission (Fig. 1a). If the donor/acceptor proportion is changed to 1/1 (0.27/0.27 wt%) or 1/1.5 (0.23/0.35 wt%), only the 700 nm emission from NB appears, but lasing efficiency is reduced to 7%. Reducing the concentrations of dyes in the nanoparticles and/or the proportion of nanoparticles in the suspension progressively reduces the lasing efficiency down to 5%, when emission at 570 nm is observed from the Rh6G (Fig. 1a).
For comparison purposes we determined the lasing properties in an ethanolic solution of both dyes while maintaining the pumping conditions, the relative proportion of donor/acceptor, as well as keeping the dye concentrations equal to those used in the colloidal suspensions. An effective energy transfer was again observed, leading to laser emission from NB, centred at 695 nm, with an efficiency of 18%, which is somewhat higher than that obtained in the colloidal suspension due to the higher affinity of NB for ethanol than for the polymer.
The true extent of the importance of dye confinement is revealed by the emission photostability under laser operation (Fig. 1b); this is an important parameter of any laser dye, because it determines the usefulness of the dye when used in practical applications. NB is a rather unstable dye under laser irradiation. In fact, its laser-induced fluorescence (LIF) emission in ethanol solution (see Methods for a detailed description of the procedure used to assess the photostability of the dyes) dropped by 80% from its initial value after just 5,000 pump pulses (Fig. 1b, curve A). The addition of Rh6G donor to the ethanol solution significantly increased NB stability, with the emission at 695 nm dropping by 55% from its initial value after 50,000 pump pulses (Fig. 1b, curve B). In contrast, when the dyes were confined in nanoparticles, the emission of NB dropped by less than 10% after 50,000 pump pulses (Fig. 1b, curve C). Thus, the FRET process, together with confinement of the dye molecules in the reduced space of the nanoparticles, significantly enhances the photostability of the laser system under consideration, in an approach that is extendable to other donor/acceptor pairs. In fact, preliminary studies in our group are showing that the improvement in lasing performance can be even higher when NB is replaced by Oxazine 720 (Ox720). This dye is very unstable, and in an ethanol solution loses its LIF emission after just 3,000 pump pulses. In contrast, a mixture of Rh6G/Ox720 (0.16/0.16 wt%), confined in the same latex nanoparticles as described above, results in laser emission at 674 nm with an efficiency of 15% (similar to that obtained in the liquid phase) and demonstrates high photostability, maintaining 100% of its initial emission after 60,000 pump pulses. The complete results will be published elsewhere.
Photophysical characterization
The mechanisms of energy transfer in this system were assessed by analysing the photophysical properties of confined donor/acceptor suspensions with respect to the photophysics of each of these dyes when encapsulated alone in the nanoparticles. Encapsulation of Rh6G or NB into nanoparticles did not significantly alter their photophysical properties. The shape of their absorption spectra matched those obtained in the corresponding diluted solution, and the fluorescence quantum yields (φ) and lifetimes (τ) of the Rh6G (φ = 0.60, τ = 4.47 ns) and NB (φ = 0.47, τ = 2.53 ns) were also similar to those recorded in diluted solutions (φ = 0.77, τ = 3.85 ns for Rh6G and φ = 0.43, τ = 2.07 ns for NB in ethanol). In spite of the dye confinement, there was no sign of aggregation, even at the high dye concentrations reached inside the latex nanoparticles. The slight fluorescence lifetime enlargement observed in the colloidal suspensions with respect to the diluted solutions can be ascribed to the spontaneous emission inhibition experienced by emitters confined in subwavelength cavities (Purcell's effect)19.
In a similar way, we carried out photophysical characterization of the latex nanoparticles containing a mixture of Rh6G and NB in a 1.5/1 weight proportion. The absorption spectrum revealed the presence of both dyes (Rh6G at 533 nm and NB at 636 nm); excitation of Rh6G in a region where NB does not absorb gave rise to fluorescence emission at 560 nm, and this was followed by strong emission at 670 nm, assigned to the fluorescence of NB (Supplementary Fig. S2). Moreover, the excitation spectrum monitored at the NB emission also showed the presence of two bands, in agreement with the corresponding absorption spectrum, revealing an efficient energy transfer from the donor Rh6G to the acceptor NB. Accordingly, this process affected the fluorescence decay curve of the confined donor Rh6G (Fig. 2a) in the presence of NB, which became multiexponential and characterized by new more dominant and faster components (0.29 and 1.05 ns) than observed for Rh6G encapsulated alone in the latex nanoparticles (4.47 ns). In fact, it will be seen later that the fluorescence decay curve cannot be described simply by a multiexponential; the relation is more complex. The NB fluorescence decay curve induced by exciting the donor molecules revealed a growing component (0.33 ns), then a decay (2.63 ns), matching the curve for the NB alone inside latex nanoparticles (2.53 ns). These results indicate that the acceptor NB excited state is populated by means of the donor, via an energy transfer process, as is nicely reproduced in the time-resolved emission spectra of Fig. 2b. Taking into account the high spectral overlap between the dyes (Supplementary Fig. S1) and their photophysical behaviour, as described above, the energy transfer mechanism from Rh6G to NB inside latex nanoparticles should be Förster type via dipole–dipole coupling. As further evidence, the FRET quantum yield φFRET = 0.77 was calculated from a comparison of the fluorescence decay curves of the donor in the absence and presence of acceptors (Supplementary equation (3)).
a, Fluorescence decay curve of Rh6G in latexes in the absence (i) and presence (ii) of NB, with excitation at 470 nm and monitoring at 560 nm. b, Fluorescence spectra (normalized at the donor emission) at different delay times after donor excitation (0.06, 0.12, 0.18, 0.30 and 0.42 ns) in latex doped with Rh6G and NB. c, Experimental (dots) and modelled (lines) fluorescence decay curves of Rh6G in the presence of NB. Fits of curve I (blue) and II (red) assume homogeneous and core/surface distributions, respectively. Inset: sketch of dye distributions (Rh6G in green dots and NB in red dots) used for curves I and II.
On the other hand, there are different ways in which NB emission could be built up: by direct energy transfer from excited Rh6G molecules (hetero-FRET) or after several energy hops among the donors coexisting in the nanoparticle (homo-FRET). Furthermore, photons emitted by an excited Rh6G molecule surviving the FRET process in a distant nanoparticle could be absorbed (by undergoing a radiative energy transfer, RET, process) by another Rh6G molecule (homo-RET) or a NB acceptor (hetero-RET). The influence of such RET processes is very important at the high optical densities required to record laser action. As evidence for this, the laser spectra suggest that the total energy transfer efficiency is much higher (0.98) than the φFRET value. The presence of RET was assessed photophysically by changing the suspension net dye concentration and the recording pathway. In diluted solutions (2 µM and 1 cm pathway), where such effects are usually negligible, the influence of reabsorption–re-emission (that is, RET) was detected in the fluorescence spectra (bathochromic shift), but RET effects were insignificant when using a 0.1 cm optical pathlength in a front-face configuration. As expected, concentrated suspensions presented higher energy transfers than diluted ones, but, once the reabsorption/re-emission phenomena were mathematically corrected, the differences vanished (Supplementary Fig. S3). In view of these results, we concluded that the energy transfer in this confined Rh6G/NB system under laser operation takes place mainly via a FRET mechanism reinforced by RET processes. Nonetheless, it has to be considered that in confined systems the emission of the donor could also be quenched, to some extent, by the sole presence of an acceptor in the ‘active sphere’ of the donor21.
Theoretical analysis of FRET
From the photophysical characterization, very interesting and useful information may be obtained about the FRET process within latex nanoparticles, as well as about their thus far imprecisely known inner morphology. This latter fact is important because of its influence on dye distribution, which may deeply affect laser performance because every monomer has a different polarity and, consequently, a different affinity for the dyes.
The fluorescence decay curve of the donor in the presence of acceptors (Fig. 2a) can be used as a tool to determine the dye distribution within a nanoparticle, because the energy transfer is highly dependent on the distribution of donor–acceptor distance11. It was therefore analysed theoretically following (but modifying where needed) the treatment developed by Martinho's group11,22,23 for FRET processes in systems with spherical symmetry (see Supplementary Section S2 for a detailed description of the treatment used). We began by assuming that the nanoparticle consists of a homogeneous tert-polymer of MMA/HEMA/glycidylmethacrylate (GMA) and that the dye distributions are therefore uniform. As seen in Fig. 2c (curve I), the fluorescence decay curve computed with this assumption fits quite accurately for short times, but deviates from the experimental points for longer times, and in fact predicts a quicker deactivation. This result indicates that the dye distribution must be, to some extent, inhomogeneous.
Our initial interpretation of this inhomegeneity was that there were two different domains, because HEMA is more polar than MMA and GMA, and would therefore tend to be closer to the aqueous solution (closer to the surface) than the MMA and GMA. Because Rh6G and NB are more soluble in HEMA than in MMA and GMA, both dyes would consequently lie closer to the surface than to the centre of the nanoparticle. Hence, the latexes would demonstrate a somewhat core/shell morphology with a smooth transition from core to shell. Following a trial-and-error approach with different core/shell morphologies (Supplementary Section S2), and in view of the fit shown in Fig. 2c (curve II), we concluded that the actual distribution of dyes within the nanoparticle is intermediate between a core/shell distribution and a core/surface one. In other words, the nanoparticles have a core/thin shell morphology, consistent with the following interpretation: (i) the core consists of a homogeneous tert-polymer of MMA/HEMA/GMA, in which both dyes are homogeneously solved; (ii) the thin shell is formed of a so-called nanoparticle ‘hairy layer’ (surface roughness or fuzziness23), in which both dyes are adsorbed with a concentration different to that of the core. As we used an anionic surfactant (SDS) in the synthesis, the ‘hairy layer’ (tert-polymer/surfactant/water interface) would have a certain negative charge distribution. Hence, cationic dyes such as Rh6G (donor) and NB (acceptor) would be adsorbed on the anionic surface with the very same distribution. Experimental and theoretical studies are currently under way to gain more insights into the inner morphology of the dye-doped latex nanoparticles and its correlation with FRET-assisted laser emission.
As a final remark, one might wonder how the presence of homo-RET, hetero-RET or homo-FRET would affect the analysis and interpretation of the decay curve. The presence of homo-RET lengthens the fluorescence decay lifetime; however, hetero-RET does not modify this decay rate, but instead modifies the output donor intensity21. These effects were avoided by carefully selecting the experimental conditions (front-face configuration and diluted solutions with a 0.1 cm pathlength, or concentrated solutions with 0.001 cm pathlength). On the other hand, the presence of homo-FRET decreases the ensemble fluorescence polarization anisotropy and leads to a possible energy spread due to excitation migration24, whereupon a change in the distribution of the excited molecules could, consequently, appear. The fluorescence anisotropy measurements (Supplementary Section S3) reveal the presence of hetero-FRET, which is somewhat reduced by the presence of the acceptor, because both homo-FRET and hetero-FRET compete for the same excitation with similar transfer probabilities (Supplementary Fig. S4). Consequently, there will be an energy migration. However, because the donor molecules are confined within the nanoparticle and the excitation spread (before being absorbed by the acceptor) will not exceed a few Förster radii24, the energy will stay in the nanoparticle. In addition, given the stochastic nature of this process, the excitation distribution will be randomized, a fact that in turn justifies the assumption of a homogeneous and spherically symmetric distribution. Hence, all of the aforementioned processes have already been taken into account in the decay curve analysis and do not call into question the reasoning about the core/shell distribution of the dyes in the nanoparticles.
Random lasing in solid samples
As a proof of concept, we show that the high efficiency of the energy transfer process in these systems is maintained when the nanoparticles are not contained in a suspension but are cast instead as solid monoliths. The solid samples, with a thickness of ∼100 µm, were obtained by drying 2 ml of suspension in an oven at 40 °C. Under front-face irradiation (see Methods), FRET-assisted stimulated emission was observed at 710 nm (Fig. 3a). However, when the solid sample was obtained by drying the suspension at room temperature, the efficiency of the energy transfer decreased dramatically, with only a small emission corresponding to fluorescence of Rh6G and NB (Fig. 3a).
To assess whether the differences in the emission from the two samples were related to structural changes induced by the drying method, we carried out scanning electron microscopy (SEM) analysis of the monoliths prepared by the two different approaches. This analysis revealed that the slow drying (at room temperature) induces a very homogeneous structure (Fig. 3b), whereas the faster drying (evaporation at 40 °C) leads to nanoparticle agglomeration and a nanostructured and heterogeneous system (Fig. 3c). Better quality and higher-resolution SEM images could not be obtained because the voltage required to do so led to clear sample degradation and modification as a result of the ‘soft’ nature of the polymeric nanoparticles.
This correlation can be understood in a random lasing picture. In random lasers, feedback is not provided by external means (defined cavity), but by multiple scattering inside the laser medium25. Incoherent random lasers, in which there is only intensity or energy feedback26, demonstrate a smooth spectral shape that is characteristic of amplified spontaneous emission (ASE) reinforced by the enlarged optical path induced by the scattering. Coherent random lasers, in which there is field or amplitude feedback26, have similar ASE spectra, but superimposed with multiple very narrow spikes associated with spatially extended lasing modes27. In our fast-dried sample, the scattering strength due to induced disorder is much higher than in the ambient-dried one due to the presence of nanoparticle aggregation; accordingly, the stimulated emission in the fast-dried sample will be reinforced by light scattering, with random lasing taking place. Taking into account that the emission spectra of this sample show no spikes, the random system would be acting in an incoherent regime. This result represents the first demonstration of FRET-assisted incoherent random lasing from solid samples of dye-doped latex nanoparticles, and shows the importance of the drying process in influencing emission properties.
In view of these results it is clear that a deeper study of the random laser properties of this type of sample, as well as the influence of structural properties, is needed. This work is under way in our laboratories and will be published elsewhere.
Conclusion
For the first time, we have demonstrated that the confinement into subwavelength nanoparticles of laser dyes undergoing FRET may significantly enhance the laser efficiency and photostability of red-emitting laser dyes pumped at 532 nm. Carefully selected mixtures of Rh6G and NB have been used in the present study, but this approach may be extended to other donor/acceptor pairs, as has been proved with Rh6G and Ox720. A thorough photophysical study has shown that the energy transfer in the confined Rh6G/NB system takes place predominantly via FRET, but backed up by RET processes. It has also revealed that the latex nanoparticles have an unexpected core/thin shell morphology. Finally, unprecedented FRET-assisted incoherent random lasing has been observed in solid samples obtained by evaporation of the colloidal suspensions. In conclusion, our approach provides a facile and cost-effective strategy to overcome the inherent low visible absorption and poor photostability problems associated with commercial red-emitting laser dyes, avoiding the need to resort to specially engineered expensive dyes.
Methods
Materials and synthesis procedures
MMA (Aldrich, 99%) was purified with a 0.1 M sodium hydroxide solution to remove inhibitor. HEMA (Aldrich, 97%), GMA (Fluka, 97%), potassium persulfate (Sigma, 99%), sodium dodecylsulphate (SDS) (Sigma, 99%), Rhodamine 6G and Nile Blue (Exciton, laser grade) were used without further purification. Deionized water was obtained from Direct QTM 5 Millipore. Dye-doped polymeric nanoparticles were prepared by batch dispersion polymerization of MMA:HEMA:GMA monomers18, introducing a monomer mixture feed with a weight ratio 67:22:11 of those monomers into the reactor, together with dye and sodium dodecylsulphate (SDS) as colloidal stabilizer. The polymerization temperature was 65 °C and the monomer content in the feed was 7% wt of the total mass of the suspension. Free radical polymerization was initiated by a water-soluble initiator, by adding 0.5% wt of potassium persulphate (KPS) with respect to the monomer present in the feed. The donor/acceptor wt% loadings in the nanoparticles against monomer feed ranged from 0.36/0.12 wt% (3/1) to 0.23/0.35 wt% (1/1.5) for the Rh6G/NB system. For comparison purposes, nanoparticles doped with only donor (Rh6G, 0.24 wt%) and only acceptor (NB, 0.15 wt%) were also prepared.
Analytical methodology
The hydrodynamic diameters of the nanoparticles were measured by dynamic light scattering (DLS) with a 90 Plus particle analyser (Brookhaven Instruments). A qualitative indicator of particle size distribution is the relative standard deviation; values smaller than 0.1 were indicative of a fairly monodisperse samples, as verified by calibration latexes. The size distribution by volume of the sensitized nanospheres was centred at 48–50 nm and had a relative standard deviation of 0.11–0.12. The morphology and microscopic structures of the latex monoliths were observed using field-emission scanning electron microscopy (FESEM, JEOL, JSM-6700F) with an acceleration voltage of 5 kV and an emission current of 10 mA. The samples were fragments of thin films obtained after drying the suspension in an oven at 40 °C and at room temperature.
Photophysical properties
The photophysical properties were registered both in diluted aqueous suspensions (optical pathway, 1 cm; right angle) and in concentrated aqueous suspensions (optical pathway, 0.1, 0.01 and 0.001 cm; front-face configuration) of latex nanoparticles doped with only Rh6G, only NB, and with a mixture of both dyes in a 1.5:1 ratio. The diluted suspensions (∼3 × 10−6 M) were obtained by diluting the original suspensions (∼3 × 10−4 M) to a latex content of 0.03–0.04 wt%. UV–vis absorption and fluorescence spectra were recorded on a Cary 4E spectrophotometer and on a SPEX Fluorolog 3-22 spectrofluorimeter, respectively. The absorption spectra were recorded using a blank suspension of latex in water as reference. Fluorescence quantum yields (φ) were evaluated from corrected spectra, using a diluted solution (1 × 10−6 M) of Rh6G dye in water (φ = 0.59) as reference28. Radiative decay curves were registered using the time-correlated single-photon timing technique (Edinburgh Instruments, model FL920), provided with a microchannel plate (Hamamatsu C4878). Fluorescence emission was monitored at the maximum emission wavelength after excitation at 470 nm by means of a diode laser (PicoQuant, model LDH470) for Rh6G and a 600 nm LED for NB. The corrected fluorescence spectra from reabsorption/re-emission phenomena were obtained by means of a mathematical method as detailed in ref. 29. The fluorescence lifetime τ was obtained from the slope after deconvolution of the instrumental response signal from the recorded decay curves using an iterative method. The goodness of the exponential fit was controlled by statistical parameters (χ2, Durbin–Watson and analysis of the residuals).
Laser properties
Colloidal suspensions of nanoparticles incorporating dyes as well as solutions of dyes in ethanol were contained in quartz cells (optical path, 1 cm), which were carefully sealed to avoid solvent evaporation. The samples were transversely pumped at 532 nm with 5.5 mJ, 6 ns FWHM pulses from a frequency-doubled Q-switched Nd:YAG laser (Monocrom STR-2+) at a repetition rate of 10 Hz. The exciting pulses were line-focused onto the front face of the cell, providing pump fluences on the active medium of 180 mJ cm−2. The oscillation cavity (length, 2 cm) consisted of a 90% reflectivity aluminium plane mirror, with the end lateral face of the cell as output coupler. Details of the experimental set-up can be found elsewhere20. The photostability of the gain medium was evaluated by irradiating (under lasing conditions) 10 µl of the colloidal suspension of nanoparticles incorporating the dyes or 10 µl of the dye solution in ethanol. The solutions and suspensions were contained in cylindrical Pyrex tubes (height, 1 cm; inner diameter, 1 mm) that were carefully sealed. Sample photolysis was monitored by recording the LIF emission from the dye solutions and suspensions in the tubes; these were placed horizontally and excited along the axis using the same pump pulses from the Nd:YAG laser used for producing dye laser emission, as a function of the pump pulses, at a repetition rate of 10 Hz. LIF was monitored perpendicular to the exciting beam, collected by an optical fibre, imaged onto the input slit of a monochromator (Acton Research), and detected using a charge-coupled device (CCD) (SpectruMM:GS128B) and/or a photomultiplier (Hamamatsu R928). LIF was recorded by feeding the signal to a boxcar (Stanford Research, model 250) to be integrated before being digitized and processed by a computer. Each measurement was repeated at least three times. The estimated error of the energy measurements was 10% and the experimental error in the photostability measurements was estimated to be on the order of 7%. The solid samples were pumped at 532 nm with 20 ns FWHM pulses from a frequency-doubled Q-switched Nd:YAG laser (Lotis TII SL-2132) at a repetition rate of 15 Hz. The pump radiation was horizontally polarized and focused onto the sample with a spherical quartz lens (f = 15 cm), at an angle of incidence of 34° with respect to the normal to the surface of the film. The spot on the surface of the sample was elliptical, with major and minor axis of 2 mm and 1.6 mm, respectively, resulting in a pump intensity of 490 kW cm−2. An optical fibre, placed normal to the sample, collected the surface emission, which was detected and analysed with the same monochromator/CCD/boxcar system described above.
References
Duarte, F. J. & Hillman, L. W. (eds) Dye Laser Principles (Academic, 1990).
Duarte, F. J. (ed.) Tunable Lasers Handbook (Academic, 1995).
Duarte, F. J. (ed.) Tunable Lasers Applications (CRC, 2009).
Steiner R. in Applied Laser Medicine (eds Berlien, H. P. & Müller, G. H.) 101–106 (Springer-Verlag, 2003).
Backmann, U. (ed.) Lambdachorme Laser Dyes (Lambda Physik, 2000).
Förster, T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann. Phys. (Leipz.) 2, 55–75 (1948).
Förster, T. Experimentelle und theoretische Untersuchung des zwischenmolecularen Uebergangs von Electronenanregungsenergie. Z. Naturforsch. 4A, 321–327 (1949).
Tcherkasskaya, O., Spiro, J. S., Ni, S. & Winnick, A. Energy transfer in restricted geometry: polyisoprene-poly(methyl methacrylate) block copolymer. J. Phys. Chem. 100, 7114–7121 (1996).
Scott, B. J., Bartl, M. H., Wirnsberger, G. & Stucky, G. D. Energy transfer in dye-doped mesostructured composites. J. Phys. Chem. A 107, 5499–5502 (2003).
Li, K. J., Oh, J. H., Kim, Y. & Jang, J. Macroscopic parallel nanocylinder array fabrication using a simple rubbing technique. Adv. Mater. 18, 2213–2215 (2006).
Farinha, J. P. S. & Martinho, J. M. G. Resonance energy transfer in polymer nanodomains. J. Phys. Chem. C 112, 10591–10601 (2008).
Lei, J., Wang, L. & Zhang, J. Radiometric pH sensor based on mesoporous silicananoparticles and Förster resonance energy transfer. Chem. Commun. 46, 8445–8447 (2010).
Sen, T., Jana, S., Koner, S. & Patra, A. Efficient energy transfer between confined dye and Y-zeolite functionalized Au nanoparticles. J. Phys. Chem. C 114, 19667–19672 (2010).
Ma, C., Zeng, F., Huang, L. & Wu, S. FRET-based radiometric detection system for mercury ions in water with polymeric particles as scaffolds. J. Phys. Chem. B 115, 874–882 (2011).
Guo, D., Knight, T. E. & McCusker, J. K. Angular momentum conservation in dipolar energy transfer. Science 334, 1684–1687 (2011).
Wang, L., Liu, Y., Chen, F., Zhang, J. & Anpo, M. Manipulating energy transfer processes between rhodamine 6G and rhodamine B in different mesoporous hosts. J. Phys. Chem. C 111, 5541–5548 (2007).
Wu, C., Zheng, Y., Szymanski, C. & McNeill, J. Energy transfer in a nanoscale multichromophoric system: fluorescent dye-doped conjugated polymer nanoparticles. J. Phys. Chem. C 112, 1772–1781 (2008).
Enciso, E., Costela, A., García-Moreno, I., Martín, V. & Sastre, R. Conventional unidirectional laser action enhanced by eye confined in nanoparticles scatters. Langmuir 26, 6154–6157 (2010).
Martín, V. et al. Photophysical and lasing properties of rhodamine 6G confined in polymeric nanoparticles. J. Phys. Chem. C 115, 3926–3933 (2011).
Costela, A., García-Moreno, I. & Sastre, R. Polymeric solid-state dye lasers: recent developments. Phys. Chem. Chem. Phys. 5, 4745–4763 (2003).
Lakowicz, J. R. (ed.) Principles of Fluorescence Spectroscopy (Kluwer Academic/Plenum, 1999).
Yekta, A., Winnik, M. A., Farinha, J. P. S. & Martinho, J. M. G. Dipole–dipole electronic energy transfer. Fluorescence decay functions for arbitrary distributions of donors and acceptors. II. Systems with spherical symmetry. J. Phys. Chem. A 101, 1787–1792 (1997).
Farinha, J. P. S., Charreyre, M-T., Martinho, J. M. G., Winnik, M. A. & Pichot, C. Picosecond fluorescence studies of the surface morphology of charged polystyrene latex particles. Langmuir 17, 2617–2623 (2001).
Barberan-Santos, M. N., Nunes Pereira, E. J. & Martinho, J. M. G. Stochastic theory of combined radiative and nonradiative transport. J. Chem. Phys. 107, 10480–10484 (1997).
Wiersma, D. S. The physics and applications of random lasers. Nature Phys. 4, 359–367 (2008).
Cao, H. Lasing in random media. Waves Random Media 13, R1–R39 (2003).
Andreasen, J. et al. Modes of random lasers. Adv. Opt. Photon. 3, 88–127 (2011).
Lopez Arbeloa, F., Lopez Arbeloa, T. & Lopez Arbeloa, I. in Handbook of Advances Electronic and Photonic Materials and Devices Ch. 5 (Academic, 2001).
López Arbeloa, I. Fluorescence quantum yield evaluation: corrections for re-absorption and re-emission. J. Photochem. 14, 97–105 (1980).
Acknowledgements
This work was supported by the Spanish MICINN (projects TRACE2009-0144, MAT2010-20646-C04-01, MAT2010-20646-C04-04 and MAT2007-65711-C04-02). The authors thank Gobierno Vasco (IT339-10) and Universidad Complutense/Banco Santander (grant no. 921556) for financial support. L.C. thanks MICINN for a predoctoral scholarship (FPI, co-financed by Fondo Social Europeo). The authors also acknowledge technical assistance from the ICTS Microscopy National Center (UCM).
Author information
Authors and Affiliations
Contributions
L.C. conducted the theoretical FRET analysis and solid sample measurements. E.E. proposed the study of FRET phenomenology in nanoparticles, synthesized the particles and helped with the theoretical FRET analysis. V.M. contributed with sample preparation. J.B. and I.L.-A. conducted the photophysical studies. A.C. supervised and coordinated the project. I.G.-M. conducted the laser measurements and supervised and coordinated the project. L.C. and I.G.-M. coordinated the manuscript preparation. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 954 kb)
Rights and permissions
About this article
Cite this article
Cerdán, L., Enciso, E., Martín, V. et al. FRET-assisted laser emission in colloidal suspensions of dye-doped latex nanoparticles. Nature Photon 6, 621–626 (2012). https://doi.org/10.1038/nphoton.2012.201
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2012.201
This article is cited by
-
Programmable complex pumping field induced color-on-demand random lasing in fiber-integrated microbelts for speckle free imaging
Science China Information Sciences (2023)
-
Dual-wavelength switchable single-mode lasing from a lanthanide-doped resonator
Nature Communications (2022)
-
Advances in organic micro/nanocrystals with tunable physicochemical properties
Science China Materials (2022)
-
Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles
Nature Protocols (2021)
-
Tuneable red, green, and blue single-mode lasing in heterogeneously coupled organic spherical microcavities
Light: Science & Applications (2020)