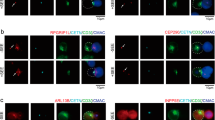
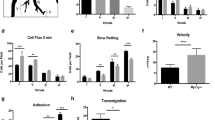
Abstract
RAPL, a protein that binds the small GTPase Rap1, is required for efficient immune cell trafficking. Here we have identified the kinase Mst1 as a critical effector of RAPL. RAPL regulated the localization and kinase activity of Mst1. 'Knockdown' of the gene encoding Mst1 demonstrated its requirement for the induction of both a polarized morphology and integrin LFA-1 clustering and adhesion triggered by chemokines and T cell receptor ligation. RAPL and Mst1 localized to vesicular compartments and dynamically translocated with LFA-1 to the leading edge upon Rap1 activation, suggesting a regulatory function for the RAPL-Mst1 complex in intracellular transport of LFA-1. Our study demonstrates a previously unknown function for Mst1 of relaying the Rap1-RAPL signal to induce cell polarity and adhesion of lymphocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Springer, T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Carman, C.V. & Springer, T.A. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15, 547–556 (2003).
van Kooyk, Y. & Figdor, C.G. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell Biol. 12, 542–547 (2000).
Dustin, M.L., Bivona, T.G. & Philips, M.R. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 5, 363–372 (2004).
Pribila, J.T., Quale, A.C., Mueller, K.L. & Shimizu, Y. Integrins and T cell-mediated immunity. Annu. Rev. Immunol. 22, 157–180 (2004).
Kinashi, T. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559 (2005).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Sanchez-Madrid, F. & del Pozo, M.A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18, 501–511 (1999).
Horton, A.C. & Ehlers, M.D. Neuronal polarity and trafficking. Neuron 40, 277–295 (2003).
Rodriguez-Boulan, E., Kreitzer, G. & Musch, A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6, 233–247 (2005).
Lawson, M.A. & Maxfield, R.R. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75–79 (1995).
Bretscher, M.S. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell 87, 601–606 (1996).
Ng, T. et al. PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 18, 3909–3923 (1999).
Bos, J.L., de Rooij, J. & Reedquist, K.A. Rap1 signaling: Adhering to new models. Nat. Rev. Mol. Cell Biol. 2, 369–377 (2001).
Shimonaka, M. et al. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J. Cell Biol. 161, 417–427 (2003).
Katagiri, K., Hattori, M., Minato, N. & Kinashi, T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol. Cell. Biol. 22, 1001–1015 (2002).
Kinashi, T. et al. LAD-III, a leukocyte adhesion deficiency syndrome associated with defective Rap1 activation and impaired stabilization of integrin bonds. Blood 103, 1033–1036 (2004).
Zemojtel, T., Penzkofer, T., Duchniewicz, M. & Zwartkruis, F.J. hRap1B-retro: a novel human processed Rap1B gene blurs the picture? Leukemia (2005).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4, 741–748 (2003).
Tommasi, S. et al. RASSF3 and NORE1: identification and cloning of two human homologues of the putative tumor suppressor gene RASSF1. Oncogene 21, 2713–2720 (2002).
Katagiri, K. et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat. Immunol. 5, 1045–1051 (2004).
Edgar, B. From cell structure to transcription: Hippo forges a new path. Cell 124, 267–273 (2006).
de Souza, P.M. & Lindsay, M.A. Mammalian sterile20-like kinase 1 and the regulation of apoptosis. Biochem. Soc. Trans. 32, 485–488 (2004).
Sells, M.A., Pfaff, A. & Chernoff, J. Temporal and spatial distribution of activated Pak1 in fibroblasts. J. Cell Biol. 151, 1449–1458 (2000).
Glantschnig, H., Rodan, G.A. & Reszka, A.A. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J. Biol. Chem. 277, 42987–42996 (2002).
Creasy, C.L., Ambrose, D.M. & Chernoff, J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J. Biol. Chem. 271, 21049–21053 (1996).
Katagiri, K. et al. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 20, 1956–1969 (2000).
Creasy, C.L. & Chernoff, J. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene 167, 303–306 (1995).
Taylor, L.K., Wang, H.C. & Erikson, R.L. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc. Natl. Acad. Sci. USA 93, 10099–10104 (1996).
Gulli, M.P. & Peter, M. Temporal and spatial regulation of Rho-type guanine-nucleotide exchange factors: the yeast perspective. Genes Dev. 15, 365–379 (2001).
Dan, I., Watanabe, N.M. & Kusumi, A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230 (2001).
Hofmann, C., Shepelev, M. & Chernoff, J. The genetics of Pak. J. Cell Sci. 117, 4343–4354 (2004).
Graves, J.D. et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 (1998).
Lee, K.K. et al. Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16, 3029–3037 (1998).
Kakeya, H., Onose, R. & Osada, H. Caspase-mediated activation of a 36-kDa myelin basic protein kinase during anticancer drug-induced apoptosis. Cancer Res. 58, 4888–4894 (1998).
Cheung, W.L. et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113, 507–517 (2003).
Khokhlatchev, A. et al. Identification of a novel Ras-regulated proapoptotic pathway. Curr. Biol. 12, 253–265 (2002).
Li, Z. et al. Directional sensing requires G βγ-mediated PAK1 and PIX α-dependent activation of Cdc42. Cell 114, 215–227 (2003).
Praskova, M., Khoklatchev, A., Ortiz-Vega, S. & Avruch, J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 381, 453–462 (2004).
Parrini, M.C., Lei, M., Harrison, S.C. & Mayer, B.J. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell 9, 73–83 (2002).
Lee, K.K., Ohyama, T., Yajima, N., Tsubuki, S. & Yonehara, S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276, 19276–19285 (2001).
Quinn, M.T., Mullen, M.L., Jesaitis, A.J. & Linner, J.G. Subcellular distribution of the Rap1A protein in human neutrophils: colocalization and cotranslocation with cytochrome b559. Blood 79, 1563–1573 (1992).
Maridonneau-Parini, I. & de Gunzburg, J. Association of Rap1 and Rap2 proteins with the specific granules of human neutrophils. Translocation to the plasma membrane during cell activation. J. Biol. Chem. 267, 6396–6402 (1992).
Pizon, V., Desjardins, M., Bucci, C., Parton, R.G. & Zerial, M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J. Cell Sci. 107, 1661–1670 (1994).
Berger, G. et al. Ultrastructural localization of the small GTP-binding protein Rap1 in human platelets and megakaryocytes. Br. J. Haematol. 88, 372–382 (1994).
Bivona, T.G. et al. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J. Cell Biol. 164, 461–470 (2004).
Fujita, H. et al. Local activation of Rap1 contributes to directional vascular endothelial cell migration accompanied by extension of microtubules on which RAPL, a Rap1-associating molecule, localizes. J. Biol. Chem. 280, 5022–5031 (2005).
Song, M.S. et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat. Cell Biol. 6, 129–137 (2004).
Fagerholm, S.C., Hilden, T.J., Nurmi, S.M. & Gahmberg, C.G. Specific integrin alpha and beta chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol. 171, 705–715 (2005).
Miyoshi, H., Smith, K.A., Mosier, D.E., Verma, I.M. & Torbett, B.E. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 283, 682–686 (1999).
Katayama, K. et al. RNA interfering approach for clarifying the PPAR γ pathway using lentiviral vector expressing short hairpin RNA. FEBS Lett. 560, 178–182 (2004).
Acknowledgements
We thank N. Shimomura, C. Tanaka and R. Hamaguchi for technical assistance. Supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan; Uehara Memorial Foundation; and Toray Science Foundation.
Author information
Authors and Affiliations
Contributions
K.K. and T.K. contributed to discussions of experimental design, data analysis and manuscript preparation and did all experimental studies unless otherwise indicated; M.I. did immunoelectron microscopy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Localization with Mst1 in T cells. (PDF 452 kb)
Supplementary Fig. 2
Pseudo-color image of LFA-1 intensities of Z-stack confocal images of the cell (asterisk) indicated in Fig. 3(d). (PDF 353 kb)
Supplementary Fig. 3
Affinity regulation by Mst1. (PDF 692 kb)
Supplementary Fig. 4
Co-localization analysis. (PDF 337 kb)
Supplementary Fig. 5
Localization of Mst1 in immunological synapse. (PDF 372 kb)
Supplementary Fig. 6
Immunoelectron microscopy of RAPL in 3A9 T cells. (PDF 1735 kb)
Supplementary Fig. 7
Relocation of MST-mRFP toward the leading edge. (PDF 471 kb)
Supplementary Fig. 8
Immunostaining for tubulin. (PDF 377 kb)
Supplementary Fig. 9
Distribution of β2-mRFP. (PDF 423 kb)
Rights and permissions
About this article
Cite this article
Katagiri, K., Imamura, M. & Kinashi, T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol 7, 919–928 (2006). https://doi.org/10.1038/ni1374
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1374
This article is cited by
-
Rap1 prevents colitogenic Th17 cell expansion and facilitates Treg cell differentiation and distal TCR signaling
Communications Biology (2022)
-
Molecular remission at T cell level in patients with rheumatoid arthritis
Scientific Reports (2021)
-
TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection
Nature Communications (2021)
-
Phosphatidic acid-dependent localization and basal de-phosphorylation of RA-GEFs regulate lymphocyte trafficking
BMC Biology (2020)
-
Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine
Nature Reviews Drug Discovery (2020)