Abstract
Asthma is a very complex and heterogeneous disease that is characterized by airway inflammation and airway hyper-reactivity (AHR). The pathogenesis of asthma is associated with environmental factors, many cell types, and several molecular and cellular pathways. These include allergic, non-allergic and intrinsic pathways, which involve many cell types and cytokines. Animal models of asthma have helped to clarify some of the underlying mechanisms of asthma, demonstrating the importance of T helper type 2 (TH2)-driven allergic responses, as well as of the non-allergic and intrinsic pathways, and contributing to understanding of the heterogeneity of asthma. Further study of these many pathways to asthma will greatly increase understanding of the distinct asthma phenotypes, and such studies may lead to new therapies for this important public health problem.
Similar content being viewed by others
Main
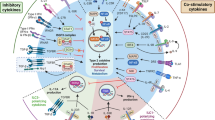
Asthma is a heterogeneous inflammatory disorder of the airways characterized by chronic airway inflammation and airway hyper-reactivity (AHR) and by symptoms of recurrent wheezing, coughing and shortness of breath. Asthma is a major public health problem, affecting 300 million people worldwide, and has increased considerably in prevalence over the past three decades in western countries1. Asthma is a complex trait caused by multiple environmental factors in combination with more than 100 major and minor susceptibility genes2,3 and has many different forms or phenotypes (Fig. 1 and Table 1). These phenotypes include allergic asthma, severe steroid-resistant asthma4 and asthma induced by exposure to air pollution5, cigarette smoke6, diesel exhaust particles7, obesity8, aspirin9 and exercise10. These different pathways and phenotypes often coexist and act in synergy in patients, although distinct pathogenic mechanisms probably underlie each of these pathways and phenotypes. In this Review we will discuss the heterogeneity of the pathogenesis of asthma phenotypes and several newly identified immunological mechanisms that underlie this disease.
Asthma is a complex disease caused by multiple factors. There are several different forms of asthma (allergic, non-allergic and intrinsic), and in some patients these forms can coexist. Allergic asthma can be induced by allergens and is mediated by TH2 immune responses. Non-allergic asthma can also be caused by several factors, such as air pollution and infection. Non-TH2 cells and various cells of the immune system other than TH2 cells contribute to non-allergic asthma. Some of the many genes involved in development of spontaneous asthma are presented here.
The classical paradigm: allergic asthma
Clinically, although several distinct forms of asthma are recognized, the major focus of treatment and research over the past 25 years has been on allergic asthma, the most common form of asthma. Allergen-specific T helper type 2 (TH2) cells are thought to be present in the lungs of almost all patients with asthma, particularly patients with allergic asthma11. TH2 cells produce cytokines that regulate the allergen-specific synthesis of immunoglobulin E (IgE; regulated by interleukin 4 (IL-4)), the recruitment of eosinophils (IL-5), the recruitment and growth of mast cells (IL-9), and AHR, a cardinal feature of asthma (IL-13)12.
Understanding of the role of allergy and TH2 cells in asthma has benefited from mouse models of allergic asthma. Allergen-specific TH2 cells can be induced in mice, and when recruited into the lungs, they cause the development of eosinophilic inflammation and AHR. However, the allergen-sensitization process in mice might not reflect the natural process that occurs in humans, which itself is poorly understood. Nevertheless, mice can be sensitized to a number of foreign proteins, such as ovalbumin (OVA), house dust mite extract or cockroach extract, as well as Aspergillus fumigatus and ragweed pollen, by immunization with adjuvants such as alum or low doses of endotoxin (or without adjuvant in the case of allergens with endogenous endotoxin or protease activity). This immunization results in a TH2-polarized response and enhanced allergen-specific IgE production. Once sensitization has occurred, repeated administration of allergen into the lungs leads to the common features of human allergic asthma, such as airway eosinophilia, mucus secretion, goblet cell hyperplasia, AHR and, when chronic, airway remodeling. In addition, adoptive transfer of allergen-specific TH2 cells generated from OVA-specific T cell antigen receptor–transgenic DO11.10 mice results in the development of AHR and airway inflammation13. In contrast, the transfer of allergen-specific TH1 cells abolishes airway eosinophilia and mucus production, although the development of AHR is not diminished14. Interestingly, newly polarized TH1 cells can produce IL-9 and IL-13 when treated with IL-18, which normally strengthens TH1 responses, particularly in combination with IL-12 (refs. 15, 16). Such TH1-TH0 cells producing IL-9 and IL-13 can then induce the development of AHR17,18. Together these results support the idea that TH2 cells producing IL-4 and IL-13 have a central role in asthma.
The induction of adaptive immunity requires antigen-presenting cells (APCs), and dendritic cells (DCs) are the main type of APC involved in the induction of TH2 responses to allergens in asthma. In the lung, DCs can be found throughout the conducting airways, interstitium, vasculature and pleura and in bronchial lymph nodes. Lung DCs express many receptors, including Toll-like receptors, Nod-like receptors and C-type lectin receptors and upregulate the expression of several costimulatory molecules (such as CD80 and CD86) and chemokines (such as CCL17 and CCL22) that attract T cells, eosinophils and basophils into the lungs19. In the OVA-induced asthma model, depletion of DCs from the airways through the use of mice transgenic for CD11c–diphtheria toxin receptor20 or thymidine kinase–transgenic mice treated with the antiviral drug ganciclovir21 abolishes the characteristic features of asthma, including eosinophilic inflammation and TH2 cytokine production. Furthermore, TH2 cells do not produce IL-4, IL-5 or IL-13 in the absence of CD11c+ DCs20, and endogenous lung DCs or adoptively transferred bone marrow–derived DCs loaded with antigen are sufficient to induce TH2 immune responses22. In humans, monocyte-derived conventional DCs promote TH2 responses by secreting proinflammatory cytokines and upregulating the expression of costimulatory molecules after antigen stimulation6. Together these findings indicate that lung DCs are the main APCs and are necessary for TH2 cell stimulation during airway inflammation.
Various inflammatory cells, such as basophils, eosinophils and mast cells, are recruited to airways after allergen challenge. Although the main focus in asthma has been on their roles as inflammatory cells, increasing data suggest that these cells also function as APCs to initiate or enhance TH2 responses (Fig. 2). Basophils, which are circulating granulocytes that express the high-affinity IgE receptor FcɛRI and the integrin CD49b (DX5) but not the stem cell factor receptor c-Kit (CD117), amplify immediate hypersensitivity responses by releasing histamine-containing granules and by producing large quantities of IL-4. Moreover, several studies have highlighted a crucial previously unknown role for basophils as APCs that drive TH2 responses through their expression of major histocompatibility complex (MHC) class II and costimulatory molecules. Culture of naive T cells (DO11.10 4get transgenic T cells) with bone marrow–derived basophils and OVA peptide in the absence of any other APC results in MHC class II–dependent TH2 differentiation23. Further, adoptive transfer of basophils into wild-type mice or Ciita−/− mice (which do not express MHC class II) followed by antigen challenge induces comparable IL-4 production from CD4+ cells, whereas antibody depletion of basophils results in less IL-4 production24,25. Although one study did not find that basophils are necessary for the development of TH2 immunity26, these results indicate that MHC class II–dependent interactions between basophils, which are prominent at sites of allergic inflammation, and CD4+ T cells might have an important role in the induction of TH2-mediated inflammation.
(a) DCs are key APCs in the lung. After antigen challenge, lung DCs process antigen and induce antigen-specific TH2 cell responses. TCR, T cell antigen receptor. (b) Other cells can also function as APCs to initiate TH2 responses. Basophils, eosinophils, mast cells and natural helper cells express MHC class II and costimulatory molecules. Therefore, these cells of the innate immune system can be the initial sources of TH2 cytokines as well as potential APCs in the lung. SCF, stem cell factor; LTC4, leukotriene C4; Lin, lineage.
Another circulating granulocyte that is prominent at sites of allergic inflammation is the eosinophil. After being stimulated, eosinophils serve an important proinflammatory role by producing cysteinyl leukotrienes, as well as TH1 cytokines (interferon-γ and IL-2) and TH2 cytokines (IL-4, IL-5, IL-10, IL-13 and tumor necrosis factor)27. In some but not all experimental models of asthma, eosinophils are required for AHR28,29, possibly due to the activity of IL-13 and cysteinyl leukotrienes and to the toxicity of eosinophil granule proteins, such as major basic protein, which contribute to airway inflammation. In addition, eosinophils, like basophils, can also function as APCs. Granulocyte-monocyte colony-stimulating factor induces the expression of MHC class II and costimulatory molecules on eosinophils30. In an OVA challenge model, antigen-loaded eosinophils have been shown to promote the production of IL-4, IL-5 and IL-13 by TH2 cells and induce IL-5 production by antigen-specific CD4+ T cells in a dose-dependent manner31. Although this APC function of eosinophils has been contested, some of the discrepancies might be explained by the methods used for isolating eosinophils that can diminish antigen processing32. Therefore, together these studies indicate that eosinophils have important effector cell functions and might also modulate adaptive TH2 immunity.
Mast cells, which are related to but distinct from basophils, express FcɛRI and c-Kit and reside in tissues near mucosal surfaces and blood vessels. Mast cells can initiate immediate hypersensitivity reactions by degranulating in response to both adaptive (IgE-mediated) and innate immune signals. For example, mast cells can be activated through crosslinking of antigen-specific IgE bound to FcɛRI (ref. 33) or in response to Toll-like receptor agonists, or with cytokines such as IL-33 (discussed below)34. In addition to producing histamine and cysteinyl leukotrienes, mast cells produce cytokines (IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-16, tumor necrosis factor and transforming growth factor-β) and chemokines (such as IL-8, lymphotactin, CCL1 (TCA-3), CCL5 (RANTES), CCL2 (MCP-1) and CCL3 (MIP1-α))35. Mast cells can enhance the development of asthma in some allergen-induced mouse models, and mast cell–deficient mouse strains (such as W/Wv, Fcer1−/− and KitW-sh/W-sh) have less AHR, airway inflammation and goblet cell hyperplasia and lower concentrations of IgE36,37. Like basophils and eosinophils, mast cells can also act as APCs, but this function is poorly understood. IL-3 (which is essential for mast cell growth), IL-4 and granulocyte-monocyte colony-stimulating factor increase mast cell expression of MHC class II and allow them to induce T cell proliferation38.
Mouse models and the complexity of asthma
The initial mouse models of allergic asthma greatly enhanced understanding of the involvement of TH2 cells and adaptive immunity in mediating allergic inflammation in asthma; however, these models reflected only one pathway to asthma (the allergen-driven pathway). Critics failing to recognize this point, however, have criticized the model as not reflecting asthma associated with “oxidant stress, viral infection, obesity and other aspects of diet and exposure to tobacco smoke, or with occupational and other pollutants”39. Furthermore, it has been suggested that the allergic model fails to recapitulate human asthma, which often “originates in early life” and with “spontaneous symptoms” that precede the development of asthma. Clearly, this experimental model of allergic asthma is not intended to reflect all forms of asthma or to replace clinical studies but instead is intended to replicate only the features of allergic asthma in humans. In this context, it is actually very similar to human allergen challenge models, as both include airway eosinophilia, AHR and dependency on TH2 cells and DCs and goblet cell metaplasia. Therefore, this model has in fact strengthened the idea that TH2 cells (which were first discovered in mouse models) and adaptive immunity can have very important roles in human asthma. In these models, mast cells are either dispensable or required, depending on how sensitization is achieved36, and eosinophils are also either dispensable (in BALB/c mice)40,41 or required (in C57BL/6 mice)28,29,42, which suggests that the function of some of the cell types can be enhanced or diminished by a number of factors, including genetic background and the strength of the signals provided by adaptive immunity. These conflicting results might reflect the heterogeneity seen in humans, in which treatments that specifically eliminate eosinophils have been effective in some but not most patients with asthma29,43. Finally, in mice repeatedly challenged with allergen, a chronic form of experimental asthma develops44 in which the relative roles of various factors, such as IL-4, eosinophils and neutrophils, change.
However, many clinical and experimental observations over the past 5 years have suggested that asthma is much more heterogeneous and complex than suggested by the TH2 paradigm and by mouse models of allergic asthma. These observations include the finding that non-TH2 factors such as interferon-γ, IL-17 and neutrophils are frequently found in the lungs of patients with asthma, particularly those with severe asthma or asthma resistant to corticosteroids. In addition, TH2-targeted therapies have not been as effective as hoped in many clinical trials of asthma45. Furthermore, nonallergic forms of asthma, triggered by environmental factors such as air pollutants (for example, smoke, diesel particles and ozone), viral infection, stress and obesity, trigger or cause asthma independently of TH2 cells5,6,7,8,46. Notably, these forms of asthma respond poorly to treatment with corticosteroids, the most common therapy for asthma, which seems to benefit mainly patients with allergic asthma. These observations suggest that asthma is indeed heterogeneous, with distinct phenotypes and with different pathogenic mechanisms, some dependent on and some independent of TH2 cells, and requiring different therapeutic approaches.
Such heterogeneity in the clinical features of asthma has spurred the development of several additional distinct experimental mouse models of asthma that can isolate and focus on specific features and molecular pathways that initiate, worsen or modulate the disease. For example, viral respiratory infection, which probably occurs independently of TH2 cells but precipitates asthma symptoms in almost all patients with asthma, regardless of the presence of allergen, has also been modeled in mice. In this model, administration of Sendai virus (a virus related to human parainfluenza), precipitates chronic lung disease associated with AHR, and this could also occur in MHC class II–deficient mice, which lack conventional CD4+ T cells and adaptive immunity46. This pathway to AHR involves alternatively activated alveolar macrophages interacting with natural killer T (NKT) cells. Evidence of such a pathway has been found in patients with severe asthma46 and in children with asthma undergoing viral infection47.
Another pathway to asthma that is independent of TH2 cells and adaptive immunity that has been successfully modeled in mice is asthma associated with air pollution. Mice repeatedly exposed to ozone, a component of air pollution, develop severe AHR associated with airway neutrophils rather than eosinophils5. The ozone-induced AHR has been shown to occur in MHC class II–deficient mice and, notably, this response requires the presence of IL-17, a neutrophil chemotactic factor, as it did not occur in Il17−/− mice. These experiments demonstrated for the first time a specific pathway to experimental asthma that requires IL-17, as allergen-induced AHR, associated with airway eosinophilia, occurred normally in the Il17−/− mice.
Intrinsic AHR is a clinical feature that can be present in some patients in whom asthma symptoms develop independently of any type of airway inflammation or of allergic sensitization. Intrinsic AHR has also been observed in a particular strain of mice: the A/J strain. These mice develop AHR spontaneously, without manipulation or treatment, independently of allergy, TH2 cells or adaptive immunity48. In addition, this intrinsic AHR is associated with a chromosomal region that contains an asthma-susceptibility gene, Adam33, first identified in A/J mice before it was recognized in humans. In humans, ADAM33 has been linked to asthma, in particular to asthma plus AHR and less so to asthma plus higher serum IgE titers or to asthma plus specific IgE49,50. Moreover, ADAM33 is expressed in bronchial smooth muscle cells, which suggests that it has an important role in intrinsic asthma, in which spontaneous AHR develops. This characteristic may allow the development of asthma symptoms in combination with any form of airway inflammation (for example, induced with allergy or infection) by converting the inflammatory effects into symptoms of asthma. Together these additional mouse models of asthma have greatly helped to solidify the idea that asthma is heterogeneous with distinct pathways that might develop independently of TH2 cells.
It should be noted that the use of linkage analysis first in mice and then in humans to identify ADAM33 and, in a similar way, to identify another asthma-susceptibility gene, HAVCR1 (TIM1)51, is an example of how mouse models can be effectively used to study human asthma. The genetic and environmental diversity seen in humans can overwhelm the ability to study specific pathogenic pathways in human patients with asthma. However, modeling of a very specific pathway through the use of reductionist methods with inbred and genetically manipulated mice can often overcome these problems, for example, by allowing single environmental factors (allergy versus oxidative stress or viral infection) to induce experimental asthma or by controlling for genetic background, for the isolation and study of specific pathophysiological effects. This is particularly advantageous when genetically manipulated mouse strains (for example, knockout or transgenic mice) are used to isolate the specific effects of single genes on asthma. Of course, the experimental results from any mouse model cannot be viewed in isolation but must be interpreted in the context of the complexity of human disease.
Innate cytokines in asthma
As noted above, adaptive immunity and TH2 cells have important roles in asthma, particularly in allergic asthma. However, it is becoming clear that innate immune mechanisms, involving a host of newly identified cytokines and cell types, can also induce AHR. Some of the pathways elicit AHR in association with TH2 cytokines, eosinphils and basophils and seem to be allergic in nature, but these other pathways can elicit AHR in the complete absence of adaptive immunity and TH2 cells. The availability of genetically manipulated mice for the study of airway disease has been key in understanding the role of several innate molecules, cytokines and target cells (Fig. 3). Many of these molecules and cells can induce AHR in mice independently of TH2 cells or adaptive immunity, which supports the concept that multiple pathways to asthma exist and that distinct asthma clinical phenotypes might be associated with specific molecular pathways. Although each of these distinct pathways can be induced and studied independently in mice, these pathways probably coexist in patients (for example, ozone- and virus-induced AHR can occur in patients with allergic asthma), which may explain why targeting a single pathway in patients is sometimes therapeutically ineffective. However, in many patients a particular pathway can predominate (for example, neutrophil- versus eosinophil-associated asthma). This gives rise to the concept of asthma phenotypes, in which a specific form of asthma predominates, such as allergic, neutrophilic or corticosteroid-resistant asthma, that responds best to a specific therapy.
Although adaptive immunity is critical for asthma pathogenesis, asthma also involves innate, antigen-independent immune responses. IL-25 induces TH2 cytokines such as IL-5 and IL-13 from natural helper cells in the absence of TH2 cells and stimulates NKT cells to produce IL-13, thereby promoting AHR and airway remodeling. IL-33 acts on multiple targets; it stimulates mast cells, eosinophils, basophils, natural helper cells and NKT cells to elicit TH2 cytokines. TSLP activates mast cells and NKT cells to secrete TH2 cytokines. The finding of these cytokines, IL-25, IL-33 and TSLP, and cells of the innate immune system greatly extends understanding of the pathogenesis of asthma.
Thymic stromal lymphopoietin (TSLP) is an IL-7-like cytokine originally cloned from a mouse thymic stromal cell line. TSLP is released by primary human epithelial cells in response to certain microbial products (such as peptidoglycan, lipoteichoic acid and double-stranded RNA), physical injury, or inflammatory cytokines (such as IL-1β and tumor necrosis factor)52. TSLP expression is detected in the airways of asthmatic patients, and TSLP mRNA expression correlates with disease severity53. In mice, TSLP mRNA expression is higher in the lungs of antigen-treated mice54. Moreover, lung-specific expression of TSLP induces airway inflammation and AHR via a pathway that requires the presence of NKT cells55. Consistent with that, TSLP receptor–deficient mice have considerably attenuated allergen-induced AHR56. TSLP can activate DCs by increasing their expression of the ligand for the T cell–costimulatory molecule OX40 and therefore enhance TH2 inflammatory responses via interactions between OX40 and its ligand57. These findings suggest that TSLP has an important role in the initiation of allergic and adaptive airway inflammation through innate pathways that initially activate airway epithelium.
IL-25 (also known as IL-17E) is a member of the IL-17 cytokine family. Both mouse and human lung epithelial lines express IL-25 after exposure to allergens, particles and helminth infection54,58,59. IL-25 is also produced by activated eosinophils, by bone marrow–derived mast cells, by basophils after FcɛRI crosslinking and by c-Kit+ cells after stimulation with stem cell factor in the lung60. More IL-25 is detected in eosinophil-infiltrated bronchial submucosa of asthmatic patients, and IL-17RB (the receptor for IL-25) is also detected in human primary lung fibroblasts61. In mouse models of asthma, several reports have shown that IL-25 amplifies TH2 cytokine production and eosinophilia. In addition, OVA sensitization and challenge in wild-type BALB/c mice results in more IL-25 mRNA in the lung. Moreover, treatment with soluble IL-25 receptor fusion protein or antibody to IL-25 before OVA sensitization and challenge results in less AHR, airway inflammation and OVA-specific serum IgE62,63. In contrast, administration of IL-25 enhances OVA-induce AHR, and IL-17RB+ NKT cells are required for development of AHR in response to IL-25 (refs. 64, 65). Furthermore, the administration of recombinant IL-25 induces production of IL-4, IL-5 and IL-13 in an innate non–B cell, non–T cell c-Kit+FcɛRI− cell population that mediates rapid expulsion of helminths in both wild-type mice and recombination-activating gene-deficient mice (discussed below)66. Together these observations indicate that IL-25 acts on both the innate and adaptive immune systems to amplify TH2 and TH2-like responses.
IL-33 is a member of the IL-1 cytokine family, and IL-33 transcripts can be detected in many diverse cell types67. IL-33 acts in synergy with stem cell factor and the IgE receptor to activate primary human mast cells and basophils34. IL-33 also enhances the survival of eosinophils and eosinophil degranulation in humans68. In mice, viral infection or exposure to pathogen-derived products, irritants or allergens can enhance IL-33 production54,69. In addition, OVA challenge induces the production of IL-33 protein in the lung, and IL-33 expression correlates with the maintenance of asthma69. When IL-33 is administered with OVA, IL-33 enhances airway inflammation in an IL-4-independent manner70. Consistent with that, administration of neutralizing antibodies to ST2 (the receptor for IL-33) or antibodies to IL-33 attenuates eosinophilic pulmonary inflammation and AHR71,72. Furthermore, the administration of IL-33 can induce AHR and even enhance airway inflammation in ST2-deficient mice73,74. Thus, IL-33 has an important role during the development of asthma in mouse models, but the specific mechanisms by which IL-33 functions in asthma are not fully understood.
IL-17 is one of the six members of the IL-17 family of cytokines (IL-17A–IL-17F). The combination of IL-6 and transforming growth factor-β skews the balance toward differentiation into IL-17-producing T helper cells (TH17 cells) by suppressing the transcription factor Foxp3 and inducing expression of RORγt, a lineage-specific transcription factor for TH17 cells75,76. In addition to TH17 cells, γδ T cells77, NKT cells78, neutrophils79 and macrophages80 produce IL-17, which is a potent neutrophil chemotactic agent. Notably, in humans with asthma, the concentration of IL-17 in sputum correlates with the severity of AHR81 and with the presence of neutrophils, which suggests an important role for IL-17. In allergic asthma, IL-17 contributes to the development of OVA-induced AHR in some but not all models. In the IL-17-dependent models, allergen sensitization through the airway, which can be enhanced by stimulation of Toll-like receptor 4, can prime strong TH17 responses that promote airway neutrophilia and acute AHR82. IL-17A-deficient or IL-17 receptor–deficient mice show impaired TH2-type allergic airway inflammation, which suggests that IL-17A contributes to the development of this form of experimental asthma83. As endotoxin induces IL-17 production, it is possible that in this model, the induction of IL-17 enhances or complements the induction of TH2 responses, which results in more AHR, although in other situations, TH2 responses alone are able to induce AHR. In the IL-17-dependent model, IL-17 could be produced by TH17 cells or by macrophages. Depleting alveolar macrophages or neutralizing IL-17 results in fewer inflammatory cells and lower concentrations of inflammatory factors in bronchoalveolar lavage (BAL) fluid80.
IL-17 is required for AHR induced in a non-allergic asthma model triggered by exposure of mice to ozone. In this model, IL-17-secreting invariant NKT (iNKT) cells are required for the induction of AHR5, which is associated with airway neutrophils. Although TH17 cells are also present in the lungs of ozone-exposed mice, the development of AHR could occur in MHC class II–deficient mice, which indicates that this response is independent of adaptive immunity and TH2 or TH17 cells. Together these studies suggest that IL-17A-secreting cells are important regulators of allergic and non-allergic asthma.
Innate effector cells in asthma
An innate lymphocyte has been identified that is present in fat-associated lymphoid clusters84 and in the mesenteric lymph nodes of helminth-infected mice85,86 and that responds to both IL-25 and IL-33. This innate cell type has been given various names, including “natural helper cells”84, “Nuocytes”85 and “multipotent progenitor cells”86. Although their precise characteristics are still being delineated, these cells do not express lineage markers but do express c-Kit, the lineage marker Sca-1, IL-7R, IL-33R and IL-17RB. After being stimulated with IL-25 or IL-33, these natural helper cells are a major source of TH2 cytokines in innate immunity. Furthermore, these cells have the potential to function as APCs because they have high expression of MHC class II and costimulatory molecules (Fig. 2). In particular, the cells described as multipotent progenitor cells can differentiate into the monocyte, macrophage, mast cell and granulocyte (eosinophil and basophil) lineages in the presence of stem cell factor and IL-3 in vitro86. Studies have described a similar non–B cell, non–T cell c-Kit+FcɛRI− cell population that produces large amounts of TH2 cytokines after IL-25 stimulation66,87. Interestingly, an innate-like non–B cell, non–T cell population has been identified after IL-25 challenge66,87, and studies have also shown that these natural helper cells produce large amounts of TH2 cytokines in response to IL-25 and IL-33 (refs. 84, 85, 86). Both IL-25 and IL-33 induce AHR without other stimulation, which suggests that natural helper cells might be one of several critical effector cells activated by IL-25 and IL-33, which mediate TH2-like responses in the lung, even in the absence of TH2 cells.
Macrophages are a major airway immunocyte and participate in lung immune homeostasis through phagocytosis and the release of mediators in response to antigens. However, their roles in asthma have not been well characterized. Originally, macrophages were reported to be negative regulators, which could modulate immune responses by inhibiting antigen presentation by DCs, T cell activation and antibody production88,89. In addition, alternatively activated macrophages (M2 cells) expressing arginase 1, chitinase-like molecules and resistin-like molecule-α (FIZZ1) can inhibit TH2 cytokine production from CD4+ cells90,91. The regulatory roles of macrophages in vivo remain controversial, but data increasingly suggest that macrophages can actively participate in asthma. Macrophages can produce both pro- and anti-inflammatory mediators, including TH1, TH2 and TH17 cytokines and IL-33, under different conditions70,92. Macrophages have been found to participate in TH2 immunity. IL-33 enhances the development of M2 cells, as Il33−/− mice show less OVA-induced airway inflammation associated with less M2 cell differentiation, and depletion of alveolar macrophages results in less IL-33-induced airway inflammation70. Additionally, CD11b+F4/80+ macrophages rather than TH17 cells produce IL-17 in some models of OVA-induced AHR80. Moreover, depletion of pulmonary macrophage inhibits the development of prolonged AHR, but depletion of either CD4+ T cells or CCR3+ eosinophils does not93. These findings emphasize the importance of macrophages as cells of the innate immune system in the development of asthma, as well as their potential relevance as a target for therapeutic intervention.
NKT cells constitute a unique subset of lymphocytes with features of both classical T cells and NK cells. The iNKT cells express a conserved or invariant T cell antigen receptor that functions as an innate pattern-recognition receptor in recognizing both foreign and endogenous glycolipid antigens presented by the MHC class I–like molecule CD1d. After being stimulated, NKT cells produce large amounts of IL-4, IL-13 and interferon-γ, which have critical roles in the regulation of immune responses94. The iNKT cells are required for the development of AHR in several mouse models of asthma, as NKT cell–deficient mice (Cd1d−/− mice, which lack NKT cells, or mice deficient in α-chain joining region 18 (Jα18), which lack the invariant T cell antigen receptor), fail to develop AHR after allergen challenge, ozone exposure or infection with Sendai virus5,46,64,95. Although the immunological pathways required for the development of AHR in these models of experimental asthma are distinct, with each requiring a phenotypically different NKT cell subset for the development of AHR96, these observations suggest that many distinct pathways to asthma require the presence of NKT cells. Moreover, innate cytokines such as IL-25, TSLP and IL-33 can stimulate NKT cells to enhance AHR. IL-17RB-expressing iNKT cells are essential for the induction of AHR, as they produce IL-13 and TH2 chemokines after being stimulated with IL-25 (refs. 64, 65). Wild-type mice depleted of IL-17RB+ NKT cells by IL-17RB-specific antibodies, as well as iNKT cell–deficient Jα18-deficient mice, fail to develop AHR after being stimulated with IL-25. In addition, adoptive transfer of IL-17RB+ iNKT cells into Jα18-deficient mice reconstitutes allergen-induced AHR64,65. These studies suggest that IL-25 can exert some of its effects via NKT cells. Other data suggest that TSLP and IL-33 might also mediate some of their effects on AHR through iNKT cells55,97,98. Together these observations indicate that iNKT cells seem to represent a common unifying element that is required for the development of AHR in several distinct models of asthma.
On the basis of animal studies, iNKT cells have also been proposed to have an important role in human asthma. The number of iNKT cells in BAL fluid, endobronchial biopsies and sputum samples from patients with asthma has been examined in several studies, and most but not all have found more iNKT cells in the lungs of asthmatics96. The difference in the number of pulmonary iNKT cells in asthmatics in different studies, although it has generated some controversy, can probably be accounted for by the heterogeneity of asthma. Thus, in one study, patients with severe, poorly controlled asthma consistently had significantly more iNKT cells in BAL fluid, whereas patients with less severe disease were less likely to have more iNKT cells in BAL fluid99. Clearly, functional studies in humans are needed to more fully assess the role of iNKT cells in human asthma. Indeed, one study has demonstrated that allergen challenge of patients with asthma results in significantly more pulmonary iNKT cells associated with significantly more AHR, which suggests that iNKT cells have an important role in at least some forms of asthma100.
Conclusions and perspective
Over the past 5 years it has become increasingly apparent that asthma is an extremely heterogeneous disease and that many clinical forms or phenotypes exist, with distinct pathogenic mechanisms. These mechanisms include allergic pathways (adaptive immunity), as well as non-allergic (innate) pathways, induced by allergen exposure, viral infection, oxidative stress and involving a now expanded list of cell types, including eosinophils, basophils, TH2 cells, DCs, neutrophils, NKT cells, natural helper cells, epithelial cells, TH17 cells and macrophages. Combined with the discovery of several previously unknown molecular pathways involving IL-25, IL-33 and TSLP, it is clear that the traditional TH2 paradigm of asthma must be extended to include many additional nontraditional pathophysiological pathways. Although these pathways can develop independently of each other, they can also coexist and interact. In humans, this interaction coupled with genetic diversity creates a complex puzzle that has stymied investigators and clinicians for decades, so that asthma remains one of the most common chronic diseases in humans. However, the recognition that asthma is heterogeneous with multiple phenotypic, molecular and cellular pathways will allow the study of asthma from a new perspective. Studies of distinct mouse and human models, informed by genetic investigation, are now underway and are being synthesized with the goal of disentangling and understanding these pathways. Such studies will probably lead to new and effective therapies for asthma that must be individualized and personalized, with the distinct pathogenic mechanisms that occur in each patient taken into account.
References
Wilson, D.H. et al. Trends in asthma prevalence and population changes in South Australia, 1990–2003. Med. J. Aust. 184, 226–229 (2006).
Umetsu, D.T., McIntire, J.J., Akbari, O., Macaubas, C. & DeKruyff, R.H. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 3, 715–720 (2002).
von Mutius, E. Gene-environment interactions in asthma. J. Allergy Clin. Immunol. 123, 3–11 (2009).
Wang, W., Li, J.J., Foster, P.S., Hansbro, P.M. & Yang, M. Potential therapeutic targets for steroid-resistant asthma. Curr. Drug Targets published online, doi:10.2174/1389210204120454501 (23 April 2010).
Pichavant, M. et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 205, 385–393 (2008).
Robays, L.J., Maes, T., Joos, G.F. & Vermaelen, K.Y. Between a cough and a wheeze: dendritic cells at the nexus of tobacco smoke-induced allergic airway sensitization. Mucosal Immunol. 2, 206–219 (2009).
Li, N., Hao, M., Phalen, R.F., Hinds, W.C. & Nel, A.E. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 109, 250–265 (2003).
Johnston, R.A. et al. Allergic airway responses in obese mice. Am. J. Respir. Crit. Care Med. 176, 650–658 (2007).
Wang, X.S., Wu, A.Y., Leung, P.S. & Lau, H.Y. PGE suppresses excessive anti-IgE induced cysteinyl leucotrienes production in mast cells of patients with aspirin exacerbated respiratory disease. Allergy 62, 620–627 (2007).
Carlsen, K.H. & Carlsen, K.C. Exercise-induced asthma. Paediatr. Respir. Rev. 3, 154–160 (2002).
Robinson, D.S. et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326, 298–304 (1992).
Holgate, S.T. & Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 8, 218–230 (2008).
Cohn, L., Homer, R.J., Marinov, A., Rankin, J. & Bottomly, K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 186, 1737–1747 (1997).
Cohn, L., Homer, R.J., Niu, N. & Bottomly, K. T helper 1 cells and interferon γ regulate allergic airway inflammation and mucus production. J. Exp. Med. 190, 1309–1318 (1999).
Walter, D.M. et al. Il-18 gene transfer by adenovirus prevents the development of and reverses established allergen-induced airway hyperreactivity. J. Immunol. 166, 6392–6398 (2001).
Maecker, H.T. et al. Vaccination with allergen-IL-18 fusion DNA protects against, and reverses established, airway hyperreactivity in a murine asthma model. J. Immunol. 166, 959–965 (2001).
Sugimoto, T. et al. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naive host mouse. J. Exp. Med. 199, 535–545 (2004).
Hayashi, N. et al. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-g and IL-13 production. Proc. Natl. Acad. Sci. USA 104, 14765–14770 (2007).
Lambrecht, B.N. & Hammad, H. Biology of lung dendritic cells at the origin of asthma. Immunity 31, 412–424 (2009).
van Rijt, L.S. et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201, 981–991 (2005).
Lambrecht, B.N., Salomon, B., Klatzmann, D. & Pauwels, R.A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J. Immunol. 160, 4090–4097 (1998).
Lambrecht, B.N. et al. Myeloid dendritic cells induce TH2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 106, 551–559 (2000).
Sokol, C.L. et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 10, 713–720 (2009).
Perrigoue, J.G. et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 10, 697–705 (2009).
Yoshimoto, T. et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 10, 706–712 (2009).
Kim, S. et al. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced TH2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 184, 1143–1147 (2010).
Akuthota, P., Wang, H.B., Spencer, L.A. & Weller, P.F. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin. Exp. Allergy 38, 1254–1263 (2008).
Lee, J.J. et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004).
Haldar, P. et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 360, 973–984 (2009).
Lucey, D.R., Nicholson-Weller, A. & Weller, P.F. Mature human eosinophils have the capacity to express HLA-DR. Proc. Natl. Acad. Sci. USA 86, 1348–1351 (1989).
MacKenzie, J.R., Mattes, J., Dent, L.A. & Foster, P.S. Eosinophils promote allergic disease of the lung by regulating CD4+ TH2 lymphocyte function. J. Immunol. 167, 3146–3155 (2001).
Rothenberg, M.E. & Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 24, 147–174 (2006).
Prussin, C. & Metcalfe, D.D. 5. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 117, S450–S456 (2006).
Silver, M.R. et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 59, 207–218 (2010).
Barrett, N.A. & Austen, K.F. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 31, 425–437 (2009).
Williams, C.M. & Galli, S.J. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J. Exp. Med. 192, 455–462 (2000).
Taube, C. et al. Mast cells, FcɛRI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J. Immunol. 172, 6398–6406 (2004).
Frandji, P. et al. Antigen-dependent stimulation by bone marrow-derived mast cells of MHC class II-restricted T cell hybridoma. J. Immunol. 151, 6318–6328 (1993).
Wenzel, S. & Holgate, S.T. The mouse trap: It still yields few answers in asthma. Am. J. Respir. Crit. Care Med. 174, 1173–1176 (2006).
Humbles, A.A. et al. A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004).
Corry, D.B. et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 183, 109–117 (1996).
Foster, P.S., Hogan, S.P., Ramsay, A.J., Matthaei, K.I. & Young, I.G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 183, 195–201 (1996).
Leckie, M.J. et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356, 2144–2148 (2000).
Johnson, J.R. et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169, 378–385 (2004).
Anderson, G.P. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 372, 1107–1119 (2008).
Kim, E.Y. et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 14, 633–640 (2008).
Subrata, L.S. et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J. Immunol. 183, 2793–2800 (2009).
Hadeiba, H., Corry, D.B. & Locksley, R.M. Baseline airway hyperreactivity in A/J mice is not mediated by cells of the adaptive immune system. J. Immunol. 164, 4933–4940 (2000).
Van Eerdewegh, P. et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature 418, 426–430 (2002).
Haitchi, H.M. et al. Induction of a disintegrin and metalloprotease 33 during embryonic lung development and the influence of IL-13 or maternal allergy. J. Allergy Clin. Immunol. 124, 590–597 (2009).
McIntire, J.J. et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2, 1109–1116 (2001).
Allakhverdi, Z. et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204, 253–258 (2007).
Ying, S. et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of TH2-attracting chemokines and disease severity. J. Immunol. 174, 8183–8190 (2005).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Nagata, Y., Kamijuku, H., Taniguchi, M., Ziegler, S. & Seino, K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int. Arch. Allergy Immunol. 144, 305–314 (2007).
Zhou, B. et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6, 1047–1053 (2005).
Seshasayee, D. et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 117, 3868–3878 (2007).
Angkasekwinai, P. et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204, 1509–1517 (2007).
Hurst, S.D. et al. New IL-17 family members promote TH1 or TH2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169, 443–453 (2002).
Dolgachev, V., Petersen, B.C., Budelsky, A.L., Berlin, A.A. & Lukacs, N.W. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived TH2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J. Immunol. 183, 5705–5715 (2009).
Letuve, S. et al. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J. Allergy Clin. Immunol. 117, 590–596 (2006).
Tamachi, T. et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 118, 606–614 (2006).
Ballantyne, S.J. et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 120, 1324–1331 (2007).
Terashima, A. et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205, 2727–2733 (2008).
Stock, P., Lombardi, V., Kohlrautz, V. & Akbari, O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J. Immunol. 182, 5116–5122 (2009).
Fallon, P.G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Liew, F.Y., Pitman, N.I. & McInnes, I.B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 10, 103–110 (2010).
Cherry, W.B., Yoon, J., Bartemes, K.R., Iijima, K. & Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 (2008).
Kearley, J., Buckland, K.F., Mathie, S.A. & Lloyd, C.M. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am. J. Respir. Crit. Care Med. 179, 772–781 (2009).
Kurowska-Stolarska, M. et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477 (2009).
Coyle, A.J. et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 190, 895–902 (1999).
Liu, X. et al. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 386, 181–185 (2009).
Kondo, Y. et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 20, 791–800 (2008).
Hoshino, K. et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 (1999).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Lockhart, E., Green, A.M. & Flynn, J.L. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4662–4669 (2006).
Michel, M.L. et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 (2007).
Li, L. et al. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120, 331–342 (2010).
Song, C. et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 181, 6117–6124 (2008).
Barczyk, A., Pierzchala, W. & Sozanska, E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir. Med. 97, 726–733 (2003).
Wilson, R.H. et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180, 720–730 (2009).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Neill, D.R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature (2010).
Saenz, S.A. et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464, 1362–1366 (2010).
Fort, M.M. et al. IL-25 induces IL-4, IL-5, and IL-13 and TH2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Bedoret, D. et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 119, 3723–3738 (2009).
Holt, P.G. et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 177, 397–407 (1993).
Nair, M.G. et al. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206, 937–952 (2009).
Pesce, J.T. et al. Arginase-1-expressing macrophages suppress TH2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371 (2009).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Yang, M., Kumar, R.K. & Foster, P.S. Interferon-γ and pulmonary macrophages contribute to the mechanisms underlying prolonged airway hyperresponsiveness. Clin. Exp. Allergy 40, 163–173 (2009).
Taniguchi, M., Harada, M., Kojo, S., Nakayama, T. & Wakao, H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21, 483–513 (2003).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 9, 582–588 (2003).
Matangkasombut, P., Pichavant, M., Dekruyff, R.H. & Umetsu, D.T. Natural killer T cells and the regulation of asthma. Mucosal Immunol. 2, 383–392 (2009).
Bourgeois, E. et al. The pro-TH2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-γ production. Eur. J. Immunol. 39, 1046–1055 (2009).
Smithgall, M.D. et al. IL-33 amplifies both Th1- and TH2-type responses through its activity on human basophils, allergen-reactive TH2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Matangkasombut, P. et al. Natural killer T cells in the lungs of patients with asthma. J. Allergy Clin. Immunol. 123, 1181–1185 (2009).
Reynolds, C. et al. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J. Allergy Clin. Immunol. 124, 860–862 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kim, H., DeKruyff, R. & Umetsu, D. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 11, 577–584 (2010). https://doi.org/10.1038/ni.1892
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1892
This article is cited by
-
Akebia saponin D attenuates allergic airway inflammation through AMPK activation
Journal of Natural Medicines (2024)
-
Airway and Systemic Immunoglobulin Profiling and Immune Response in Adult Asthma
Lung (2024)
-
Chronic allergic asthma induces T-cell exhaustion and impairs virus clearance in mice
Respiratory Research (2023)
-
Isthmin-1 attenuates allergic Asthma by stimulating adiponectin expression and alveolar macrophage efferocytosis in mice
Respiratory Research (2023)
-
Association between PFAS congeners exposure and asthma among US children in a nationally representative sample
Environmental Geochemistry and Health (2023)