Abstract
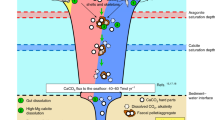
Carbon is removed from the Earth’s surface through the formation and burial of carbon-bearing rocks and minerals1,2. The formation of calcium carbonate and its burial in marine sediments accounts for around 80% of the total carbon removed from the Earth’s surface. However, the fraction of calcium carbonate that precipitates in the oceans, versus that which precipitates authigenically in marine sediments, is unclear. Here, we compile measurements of the calcium concentration of pore fluids collected at 672 seafloor sites around the globe to calculate the global flux of calcium within marine sediments. We use these data, combined with alkalinity measurements of pore fluids, to quantify authigenic calcium carbonate precipitation. We estimate that the net calcium flux into marine sediments that can be ascribed to authigenic carbonate precipitation amounts to around 1×1012 mol yr−1. As such, we estimate that authigenic carbonate precipitation accounts for at least 10% of global carbonate accumulation. We show that much of the precipitation occurs along the eastern margins of ocean basins, where organic matter delivery to the sea floor is likely to be high. We suggest that authigenic calcium carbonate precipitation represents a non-negligible component of the global carbon cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Berner, R. A. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature 426, 323–326 (2003).
Archer, D. The Global Carbon Cycle (Princeton Univ. Press, 2010).
Milliman, J. D. Production and accumulation of calcium carbonate in the ocean: Budget of a nonsteady state. Glob. Biogeochem. Cycles 7, 927–957 (1993).
Falkowski, P. et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 290, 291–296 (2000).
Milliman, J. D. & Droxler, A. W. Neritic and pelagic carbonate sedimentation in the marine environment: Ignorance is not bliss. Geol. Rundsch. 85, 496–504 (1996).
Burdige, D. J., Hu, X. & Zimmerman, R. C. The widespread occurrence of coupled carbonate dissolution/reprecipitation in surface sediments on the Bahamas Bank. Am. J. Sci. 310, 492–521 (2010).
Froelich, P. N. et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim. Cosmochim. Acta 43, 1075–1090 (1979).
Berner, R. A., Scott, M. R. & Thomlinson, C. Carbonate alkalinity in the pore waters of anoxic marine sediments. Liminol. Oceanogr. 12, 365–368 (1970).
Berner, R. A. Early Diagenesis: A Theoretical Approach (Princeton Univ. Press, 1980).
Higgins, J. A., Fischer, W. W. & Schrag, D. P. Oxygenation of the ocean and sediments: Consequences for the seafloor carbonate factory. Earth Planet. Sci. Lett. 284, 25–33 (2009).
Schrag, D. P., Higgins, J. A., Macdonald, F. A. & Johnston, D. T. Authigenic carbonate and the history of the global carbon cycle. Science 339, 540–543 (2013).
Ben-YaaKov, S. pH buffering of pore water of recent anoxic marine sediments. Limnol. Oceanogr. 18, 86–94 (1973).
Zeebe, R. E. Modeling CO2 chemistry, δ13C, and oxidation of organic carbon and methane in sediment porewater: Implications for paleo-proxies in benthic foraminifera. Geochim. Cosmochim. Acta 71, 3238–3256 (2007).
Smith, S. V. & Hollibaugh, J. T. Coastal metabolism and the oceanic organic carbon balance. Rev. Geophys. 31, 75–89 (1993).
Fantle, M. S. & DePaolo, D. J. Ca isotopes in carbonate sediment and pore fluid from ODP Site 807A: The Ca2 +(aq)–calcite equilibrium fractionation factor and calcite recrystallization rates in Pleistocene sediments. Geochim. Cosmochim. Acta 71, 2524–2546 (2007).
Walter, L. M., Ku, T. C. W., Muehlenbachs, K., Patterson, W. P. & Bonnell, L. Controls on the δ13C of dissolved inorganic carbon in marine pore waters: An integrated case study of isotope exchange during syndepositional recrystallization of biogenic carbonate sediments (South Florida Platform, USA). Deep Sea Res. II 54, 1163–1200 (2007).
Morse, J. W., Gledhill, D. K. & Millero, F. J. CaCO3 precipitation kinetics in waters from the great Bahama bank: Implications for the relationship between bank hydrochemistry and whitings. Geochim. Cosmochim. Acta 67, 2819–2826 (2003).
Sayles, F. L. The composition and diagenesis of interstitial solutions—I. Fluxes across the seawater-sediment interface in the Atlantic Ocean. Geochim. Cosmochim. Acta 43, 527–545 (1979).
Sayles, F. L. The composition and diagenesis of interstitial solutions—II. Fluxes and diagenesis at the water-sediment interface in the high latitude North and South Atlantic. Geochim. Cosmochim. Acta 45, 1061–1086 (1981).
Amiotte Suchet, P., Probst, J-L. & Ludwig, W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob. Biogeochem. Cycles 17, 1038 (2003).
Soetaert, K., Hofmann, A. F., Middelburg, J. J., Meysman, F. J. R. & Greenwood, J. Reprint of the effect of biogeochemical processes on pH. Mar. Chem. 106, 380–401 (2007).
Boudreau, B. P. & Canfield, D. E. A comparison of closed- and open-system models for porewater pH and calcite-saturation state. Geochim. Cosmochim. Acta 57, 317–334 (1993).
Joseph, C. et al. Methane-derived authigenic carbonates from modern and paleoseeps on the Cascadia margin: Mechanisms of formation and diagenetic signals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 390, 52–67 (2013).
Boudreau, B. P. Diagenetic Models and Their Implementation: Modelling Transport and Reactions in Aquatic Sediments (Springer, 1997).
Ullman, W. J. & Aller, R. C. Diffusion coefficients in nearshore marine sediments. Liminol. Oceanogr. 27, 552–556 (1982).
Zeebe, R. E. On the molecular diffusion coefficients of dissolved CO2, HCO3 and CO32− and their dependence on isotopic mass. Geochim. Cosmochim. Acta 75, 2483–2498 (2011).
Acknowledgements
This work was supported by an ERC Starting Investigator Grant (307582) to A.V.T. J. A. A. Dickson read this paper before submission and his comments greatly improved the manuscript.
Author information
Authors and Affiliations
Contributions
A.V.T. conceived this project. X.S. conducted the calculations and data analysis. Both authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 416 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Turchyn, A. Significant contribution of authigenic carbonate to marine carbon burial. Nature Geosci 7, 201–204 (2014). https://doi.org/10.1038/ngeo2070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2070
This article is cited by
-
Aragonite dissolution protects calcite at the seafloor
Nature Communications (2022)
-
Sulfur bacteria promote dissolution of authigenic carbonates at marine methane seeps
The ISME Journal (2021)
-
Mantle data imply a decline of oxidizable volcanic gases could have triggered the Great Oxidation
Nature Communications (2020)
-
The role of marine sediment diagenesis in the modern oceanic magnesium cycle
Nature Communications (2019)
-
Organic and inorganic carbon and their stable isotopes in surface sediments of the Yellow River Estuary
Scientific Reports (2018)