Abstract
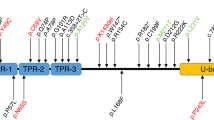
The past decade has seen great advances in unraveling the biological basis of hereditary ataxias. Molecular studies of spinocerebellar ataxias (SCA) have extended our understanding of dominant ataxias1. Causative genes have been identified for a few autosomal recessive ataxias: Friedreich's ataxia2, ataxia with vitamin E deficiency3, ataxia telangiectasia4, recessive spastic ataxia of Charlevoix-Saguenay5 and ataxia with oculomotor apraxia type 1 (refs. 6,7) and type 2 (ref. 8). Nonetheless, genes remain unidentified for most recessive ataxias. Additionally, pure cerebellar ataxias, which represent up to 20% of all ataxias, remain poorly studied with only two causative dominant genes being described: CACNA1A (ref. 9) and SPTBN2 (ref. 10). Here, we report a newly discovered form of recessive ataxia in a French-Canadian cohort and show that SYNE1 mutations are causative in all of our kindreds, making SYNE1 the first identified gene responsible for a recessively inherited pure cerebellar ataxia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schols, L., Bauer, P., Schmidt, T., Schulte, T. & Riess, O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 3, 291–304 (2004).
Campuzano, V. et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996).
Ouahchi, K. et al. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat. Genet. 9, 141–145 (1995).
Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749–1753 (1995).
Engert, J.C. et al. ARSACS, a spastic ataxia common in northeastern Quebec, is caused by mutations in a new gene encoding an 11.5-kb ORF. Nat. Genet. 24, 120–125 (2000).
Date, H. et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat. Genet. 29, 184–188 (2001).
Moreira, M.C. et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat. Genet. 29, 189–193 (2001).
Moreira, M.C. et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat. Genet. 36, 225–227 (2004).
Zhuchenko, O. et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat. Genet. 15, 62–69 (1997).
Ikeda, Y. et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 38, 184–190 (2006).
Apel, E.D., Lewis, R.M., Grady, R.M. & Sanes, J.R. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 275, 31986–31995 (2000).
Grady, R.M., Starr, D.A., Ackerman, G.L., Sanes, J.R. & Han, M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 102, 4359–4364 (2005).
Sanes, J.R. & Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 (2001).
Starr, D.A. & Han, M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298, 406–409 (2002).
Zhang, Q., Ragnauth, C., Greener, M.J., Shanahan, C.M. & Roberts, R.G. The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics 80, 473–481 (2002).
Kamath, R.S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003).
Rosenberg-Hasson, Y., Renert-Pasca, M. & Volk, T. A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech. Dev. 60, 83–94 (1996).
Cottrell, J.R., Borok, E., Horvath, T.L. & Nedivi, E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron 44, 677–690 (2004).
Koenig, M. et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50, 509–517 (1987).
Ishikawa, K. et al. An autosomal dominant cerebellar ataxia linked to chromosome 16q22.1 is associated with a single-nucleotide substitution in the 5′ untranslated region of the gene encoding a protein with spectrin repeat and Rho guanine-nucleotide exchange-factor domains. Am. J. Hum. Genet. 77, 280–296 (2005).
Parkinson, N.J. et al. Mutant beta-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nat. Genet. 29, 61–65 (2001).
Holmes . A form of familial degeneraton of the cerebellum. Brain 30, 466 (1908).
Acknowledgements
The authors thank all family members for their entire cooperation. The authors also thank D. Verlaan and P. Cossette for helpful discussion on the linkage study, C. Gaspar for careful review of this manuscript, P. Hince for technical assistance with the immunohistological experiments, F. Gosselin for collecting blood from affected individuals and N. Chrestian for data acquisition and management. We are grateful for the support of T. Hudson from the McGill University Genome Québec Innovation Centre. We thank P. Greengard for antibody to synapsin. F.G.L., N.D. and G.A.R. are supported by the Canadian Institutes of Health Research (CIHR). This project was funded by the Canadian Genetic Diseases Network (CGDN), by the US National Ataxia Foundation and by a grant from the US National Institutes of Health to J.R.S.
Author information
Authors and Affiliations
Contributions
F.G.-L. generated the data, conducted the data analysis, wrote the manuscript and led the project; N.D. conducted neurological evaluation of individuals with ARCA1, described the ARCA1 phenotype and reviewed the manuscript; P.D. participated in the data analysis and review of the manuscript; M.A.F. conducted and analyzed in vitro neuromuscular junction experiments and reviewed the manuscript; S.L. provided technical assistance in generating data; S.V. participated in the neurological evaluation of individuals with ARCA1; J.R.S. supervised and analyzed the in vitro neuromuscular junction experiments and reviewed the manuscript; J.-P.B. conducted neurological evaluation of individuals with ARCA1, described the ARCA1 phenotype and reviewed the manuscript; G.A.R. conducted neurological evaluation of individuals with ARCA1, described the ARCA1 phenotype, participated in the data analysis, reviewed the manuscript and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
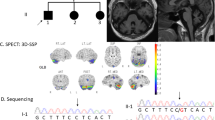
Magnetic resonance imaging of affected subjects in sagittal T1 and axial T2 showing marked diffuse cerebellar atrophy with no cortical or brainstem atrophy. (PDF 148 kb)
Supplementary Fig. 2
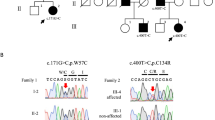
Pedigrees with autosomal recessive cerebellar ataxia type 1 used for the genome-wide scan analysis. (PDF 52 kb)
Supplementary Table 1
Genome-wide linkage results. (PDF 49 kb)
Supplementary Table 2
Details, location, and genomic context of each variant detected. (PDF 14 kb)
Supplementary Table 3
PCR primer pair sequences. (PDF 43 kb)
Rights and permissions
About this article
Cite this article
Gros-Louis, F., Dupré, N., Dion, P. et al. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet 39, 80–85 (2007). https://doi.org/10.1038/ng1927
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1927
This article is cited by
-
Novel Homozygous Truncating Variant Widens the Spectrum of Early-Onset Multisystemic SYNE1 Ataxia
The Cerebellum (2022)
-
Autosomal recessive adult onset ataxia
Journal of Neurology (2022)
-
Eye-tracking-aided characterization of saccades and antisaccades in SYNE1 ataxia patients: a pilot study
BMC Neuroscience (2021)
-
The N-terminal region of Jaw1 has a role to inhibit the formation of organized smooth endoplasmic reticulum as an intrinsically disordered region
Scientific Reports (2021)
-
Autosomal Recessive Cerebellar Ataxia Type 1: Phenotypic and Genetic Correlation in a Cohort of Chinese Patients with SYNE1 Variants
The Cerebellum (2021)