Abstract
X-chromosome inactivation (XCI) in mammals relies on XIST, a long noncoding transcript that coats and silences the X chromosome in cis. Here we report the discovery of a long noncoding RNA, XACT, that is expressed from and coats the active X chromosome specifically in human pluripotent cells. In the absence of XIST, XACT is expressed from both X chromosomes in humans but not in mice, suggesting a unique role for XACT in the control of human XCI initiation.
Similar content being viewed by others
Main
A major recent breakthrough in the conception of eukaryotic gene regulation has been the identification of a multitude of long noncoding RNAs (lncRNAs) in mammals that are scattered throughout the genome and are located, in particular, in intergenic regions (lincRNAs)1. Although the number of lncRNAs is estimated to be more than 1,000, only 12 have been functionally characterized. These lncRNAs were shown to function in diverse cellular processes, most predominantly in the regulation of gene expression. In this context, many lncRNAs participate in gene silencing pathways, some of which involve the recruitment of repressive chromatin complexes2.
XCI is one of the most studied processes involving lncRNA-mediated repression. XIST is a noteworthy transcript in that in addition to silencing an (approximate) entire chromosome, it is the only lncRNA described thus far to widely coat the chromosome from which it is expressed. This coating by XIST induces substantial nuclear reorganization and recruitment of histone-modifying complexes that are important for the initiation and maintenance, respectively, of X-chromosome silencing3.
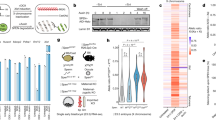
XCI is established early during embryonic development, and embryonic stem cells can be used to decipher the kinetics and molecular actors of the process. In female human embryonic stem cells (hESCs), one X chromosome is, in most cases, already inactivated4. Using an RNA sequencing (RNA-seq) analysis of female H9 hESCs (C.V., Ouimette, J.-F., Makhlouf, M., Féraud, O., N.O., A.B.-G., Côme, J., Martinat, C., A.R., Lalande, M. & C.R., unpublished data), we identified a large unannotated region (∼252 kb) on the X chromosome for which we could detect a substantial amount of expression originating from the minus strand (Fig. 1a). We called this transcript XACT. The XACT region is located on chromosome Xq23 between the protein-coding genes AMOT and HTR2C in an unusually large intergenic domain of 1.7 Mb (only 1% of intergenic regions in humans are >1.5 Mb). This domain is GC poor (37%) and rich in repeat sequences (68% compared to 62% for the X chromosome and 45% for the overall human genome), notably long interspersed nuclear elements and long terminal repeats (LTRs) (Supplementary Fig. 1). All RNA-seq reads were co-linear with the genomic sequence, suggesting that the XACT transcript is unspliced (Supplementary Methods). We detected similar transcription in male H1 hESCs, and alignment with published chromatin immunoprecipitation sequencing data5 revealed histone H3 Lys36 trimethylation (H3K36me3) and histone H3 Lys79 dimethylation (H3K79me2) blocks along the region, as well as peaks of H3K4me3 and RNA polymerase II (PolII) at the telomeric extremity of the transcribed region (Supplementary Fig. 2a). These peaks correspond to LTR elements, which might serve as promoters for this transcribed region6. We further characterized the 5′ end of XACT by RT-PCR and 5′ RACE (Supplementary Fig. 2b). This analysis revealed multiple transcription start sites (TSSs) clustered in a 126-bp region characteristic of broad-type promoters7, which coincide with peaks of H3K4me3 and RNA PolII (Supplementary Fig. 2). Together these data suggest that XACT corresponds to a single 251.8-kb transcription unit (112,983,323–113,235,148 bp). The XACT transcript is polyadenylated and mostly nuclear (Fig. 1b), further suggesting that it acts, at least in part, as a noncoding RNA. We also identified a distinct transcript 5′ of XACT on the plus strand called T113.3 (Fig. 1a and Supplementary Fig. 3). Unlike XACT, T113.3 is spliced and mostly cytoplasmic, and its TSS, mapped by 5′ RACE, lies within a peak of H3K4me3 located 48 kb upstream of the TSS of XACT.
(a) Schematic map showing the localization of XACT and XIST on the human X chromosome. Bottom, strand-specific RNA-seq showing >250 kb of continuous transcription originating from the minus strand in female H9 cells. The boxed area is a magnification of the locus. The locations of the BAC probe (RP11-35D3) and fosmid probes (f1–f7) used for further FISH analysis are indicated below (Supplementary Fig. 4). Probes giving an RNA cloud signal for XACT are in red, and probes not giving a signal are in black. RPM, reads per million mapped reads. (b) Left, semiquantitative RT-PCR analysis of poly(A)-positive (A+) and poly(A)-negative (A–) enriched RNA fractions of H9 hESCs. NANOG and U6 were used as positive controls for the poly(A)-positive and poly(A)-negative fractions, respectively. Right, RT-PCR analysis of total (T), cytoplasmic (C) and nuclear (N) RNAs. (c) RNA FISH analysis of H9 cells with a XIST probe (green) and a XACT probe (red). All XACT FISH experiments were performed using the BAC RP11-35D3 probe, except where otherwise indicated. (d) Simultaneous RNA and DNA FISH of H9 cells with the XACT probe (red) detecting XACT RNA and DNA and an X-paint probe (green) detecting X chromosomes. (e) Left and middle, XACT RNA FISH with and without initial treatment with RNase. Right, XACT DNA FISH with initial treatment with RNase. Scale bars (c–e), 5 μm. (f) Three-dimensional model of the nuclear volumes occupied by XACT and XIST RNAs. The boxplot represents the distribution of the volumes (n = 50 nuclei). The distribution of the volumes corresponding to the transcription signal of an X-linked gene (ATRX) is shown for comparison. The median value of each distribution is indicated above the corresponding boxplot.
We next investigated transcription of XACT at the cellular level by RNA FISH. Remarkably, a BAC probe covering 151 kb of the transcribed region detected a unique, large signal in female H9 hESCs that was reminiscent of the XIST RNA cloud. However, this signal corresponded to the active X chromosome, as determined by combined RNA FISH with a XIST probe, which labels the inactive X chromosome (Fig. 1c), and simultaneous RNA and DNA FISH with an X-paint probe (Fig. 1d). The XACT RNA cloud signal was partially resistant to the stringent denaturation steps used in the DNA FISH experiments (Fig. 1d; note that the XACT signal appears smaller than it does in classical RNA FISH experiments) but not to RNase treatment (Fig. 1e), indicating a strong association of the RNA with the active X chromatin. The BAC probe detected two pinpoints in the DNA FISH experiments (after RNase treatment of the slides), which confirms that the RNA cloud signal corresponds to actual coating of the chromosome by XACT RNA and not to the genomic organization of the locus. We called this RNA XACT (X active coating transcript). The nuclear volume occupied by XACT in the nucleus was similar to that occupied by XIST (median volumes of 3.75 μm3 and 2.94 μm3, respectively; Fig. 1f). RNA FISH analysis of XACT expression using a series of fosmid probes covering the region (Fig. 1a) further confirmed the extent and expression profile of XACT (Supplementary Fig. 4).
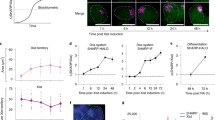
X inactivation is highly unstable in female hESCs, and XIST downregulation tends to occur spontaneously in culture8. Loss of XIST expression leads to substantial but incomplete gene reactivation from the originally inactive X chromosome (Xi*)9 (C.V., Ouimette, J.-F., Makhlouf, M., Féraud, O., N.O., A.B.-G., Côme, J., Martinat, C., A.R., Lalande, M. & C.R., unpublished data). RNA-seq performed in H9 hESCs not expressing XIST revealed a slight increase in XACT expression compared to H9 cells that did express XIST (Fig. 2a; reads per kilobase per million reads (RPKM) = 1.78 in cells expressing XIST, and RPKM = 2.37 in cells not expressing XIST). We took advantage of a SNP (rs5929175) within XACT and the clonal X-inactivation pattern shown by H9 cells10,11 to address the allelic expression of XACT in cells not expressing XIST compared to XIST-expressing cells. Whereas XACT was monoallelically transcribed in cells expressing XIST, it became biallelically expressed in cells not expressing XIST (Fig. 2b). RNA FISH analysis further revealed that XACT is not only re-expressed from but also coats the Xi* in cells not expressing XIST, leading to two XACT clouds in these cells (Fig. 2b). Whether XACT re-expression from and coating of the Xi* is a cause or a consequence of XIST repression and subsequent partial reactivation of the chromosome remains to be established.
(a) RNA-seq data for H9 hESCs expressing (XIST+) or not expressing (XIST–) XIST with RPKM values shown. (b) Left, pyrosequencing analysis of rs5929175 for H9 genomic DNA (gDNA) and XIST+ and XIST– complementary DNA. The bar chart indicates the percentage of the peak height corresponding to each allele. Right, RNA FISH using the XACT probe in XIST– H9 hESCs revealing that 79% of the nuclei (n = 150) have two XACT RNA clouds. (c) RNA FISH and quantitative RT-PCR analysis of XACT expression during differentiation of XIST– hESCs and in female fetal (IMR90) and adult (Coriell, AG09603) fibroblasts. d0–d10, days 0–10. The images are representative of the major population. The bar charts correspond to the average of two independent differentiation experiments. Errors bars indicate the s.d. calculated for two samples. (d) RNA FISH and pyrosequencing analysis of XACT expression during successive rounds of differentiation and reprogramming of XIST– H9 cells into MSCs. Scale bars (b–d), 5 μm. The percentages shown in b–d indicate the number of nuclei showing similar expression patterns of XACT.
XACT is expressed from active X chromosome(s) in hESCs. In contrast, we were not able to detect XACT reads from female fibroblast RNA-seq libraries. Similarly, RT-PCR analysis revealed no or weak expression of XACT in various tissues, including brain, muscle and placenta (Supplementary Fig. 5), suggesting that XACT is downregulated after differentiation. To investigate the kinetics of XACT silencing, we induced H9 hESCs to differentiate and found substantial downregulation of XACT at day 5 and full silencing at day 10 (Fig. 2c). To further probe the link between XACT and pluripotency, we used a model system in which H9 hESCs not expressing XIST were subjected to several rounds of differentiation and reprogramming12 (Fig. 2d). As detected with undirected differentiation, the differentiation of these H9 hESCs into mesenchymal stem cells (MSCs) was accompanied by biallelic silencing of XACT. Remarkably, we found strong re-expression of XACT from both X chromosomes in MSC-derived induced pluripotent stem (iPS) cells, whereas silencing of XACT was re-established when these iPS cells were differentiated into MSCs (iPS-MSCs). Together these results indicate that, in this context, XACT expression and coating of the X chromosomes is restricted to pluripotent and early differentiating cells in humans. However, we cannot exclude the possibility that XACT is expressed in some differentiated cell types.
We then investigated the conservation of XACT by combining in silico analyses and experimental studies (Supplementary Fig. 6). The organization of the genomic region encompassing AMOT and HTR2C is well conserved in placental mammals and marsupials. In contrast, the sequence between these two genes shows moderate conservation, with several conserved blocks present in placental mammals but not marsupials, which are interspaced with divergent regions. The LTR that corresponds to the 5′ end of XACT is conserved in chimpanzees but not macaques or more distally related species (Supplementary Fig. 6a). This suggests that the insertion of these LTR elements is a very recent event. To further probe XACT conservation in mice, we undertook systematic DNA and RNA FISH studies in mouse ESCs (mESCs) using a series of six BAC probes spanning the region between Amot and Htr2c. Although all these probes detected the two X chromosomes by DNA FISH, none of them generated a signal by RNA FISH (Supplementary Fig. 6b), suggesting that the region syntenic to XACT is not expressed in mESCs. In agreement with this result, analysis of mESC RNA-seq data did not reveal broad, 'XACT-like' transcription in the syntenic region. However, we were able to identify a few discrete peaks in mESCs and other cell types (Supplementary Fig. 6b). Whether these peaks correspond to real transcripts remains to be investigated. Together our data suggest that there is no XACT-like transcript expressed in mESCs.
We have identified XACT as the first lncRNA that coats the active X chromosome in humans. The identification of a lncRNA that coats an active chromosome (whereas most lncRNAs studied so far are involved in gene silencing) underlies the multifaceted nature of lncRNAs. XACT might not be conserved in mice, and its function might be related to the specific kinetics of XCI that were recently described in the human. Indeed, in human preimplantation embryos, XIST is expressed from the paternal and maternal X chromosomes, but this does not lead to chromosome-wide silencing13. In contrast, paternal Xist expression and XCI characterize mouse preimplantation development14. Given its expression profile, it is tempting to speculate that XACT is involved in the control of XCI initiation in humans. More generally, rapidly evolving lncRNAs such as XACT in the human and Tsix in the mouse15 could be involved in species-specific regulation of XCI, thus underlying the important plasticity that characterizes this process among mammals.
Accession codes. Sequence data and alignments have been submitted to the Gene Expression Omnibus (GEO) database under accession code GSE39757.
Accession codes
References
Guttman, M. et al. Nature 458, 223–227 (2009).
Khalil, A.M. et al. Proc. Natl. Acad. Sci. USA 106, 11667–11672 (2009).
Augui, S., Nora, E.P. & Heard, E. Nat. Rev. Genet. 12, 429–442 (2011).
Dvash, T. & Fan, G. Epigenetics 4, 19–22 (2009).
The ENCODE Project Consortium. PLoS Biol. 9, e1001046 (2011).
Romanish, M.T., Lock, W.M., van de Lagemaat, L.N., Dunn, C.A. & Mager, D.L. PLoS Genet. 3, e10 (2007).
Sandelin, A. et al. Nat. Rev. Genet. 8, 424–436 (2007).
Silva, S.S., Rowntree, R.K., Mekhoubad, S. & Lee, J.T. Proc. Natl. Acad. Sci. USA 105, 4820–4825 (2008).
Mekhoubad, S. et al. Cell Stem Cell 10, 595–609 (2012).
Mitjavila-Garcia, M.T. et al. J. Mol. Cell Biol. 2, 291–298 (2010).
Shen, Y. et al. Proc. Natl. Acad. Sci. USA 105, 4709–4714 (2008).
Giuliani, M. et al. Blood 118, 3254–3262 (2011).
Okamoto, I. et al. Nature 472, 370–374 (2011).
Okamoto, I., Otte, A.P., Allis, C.D., Reinberg, D. & Heard, E. Science 303, 644–649 (2004).
Rougeulle, C. & Avner, P. Semin. Cell Dev. Biol. 14, 331–340 (2003).
Acknowledgements
We thank members of our laboratory and M. Lalande for stimulating discussion and critical reading of the manuscript. The research leading to these results has received funding from the European Research Council (ERC) under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement 206875 and the INSERM (Avenir Program R0721HS).
Author information
Authors and Affiliations
Contributions
C.V. and C.H. performed the experiments. Y.L. and L.D. did the bioinformatic analysis of XACT conservation. N.O. and A.B.-G. provided the H9-MSC-iPS system. A.R. contributed to the bioinformatics analysis of the RNA-seq data. C.V. and C.R. conceived and designed the experiments, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–6 (PDF 9036 kb)
Rights and permissions
About this article
Cite this article
Vallot, C., Huret, C., Lesecque, Y. et al. XACT, a long noncoding transcript coating the active X chromosome in human pluripotent cells. Nat Genet 45, 239–241 (2013). https://doi.org/10.1038/ng.2530
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2530
This article is cited by
-
Epigenetic control of chromosome-associated lncRNA genes essential for replication and stability
Nature Communications (2022)
-
Preventing erosion of X-chromosome inactivation in human embryonic stem cells
Nature Communications (2022)
-
Gene regulation in time and space during X-chromosome inactivation
Nature Reviews Molecular Cell Biology (2022)
-
Many XCI-ting routes to reach the eXACT dose
Nature Cell Biology (2020)
-
Female human primordial germ cells display X-chromosome dosage compensation despite the absence of X-inactivation
Nature Cell Biology (2020)