Article
|
Open Access
Featured
-
-
Article
| Open Access Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants
Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants
Photosynthesis declines at mild temperatures in terrestrial plants. Here, the authors use published data to show that decline in photosynthetic CO2 assimilation rate with rising temperatures can be accounted for by Rubisco deactivation and declines in chloroplast electron transport rate.
- Andrew P. Scafaro
- , Bradley C. Posch
- & Owen K. Atkin
-
Article
| Open Access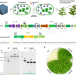 Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis
Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis
Engineering carboxysomes into crop chloroplasts is a potential route to improve photosynthesis and crop yield. Here, the authors engineer functional CO2-fixing modules into tobacco chloroplasts to improve their photosynthesis and productivity.
- Taiyu Chen
- , Marta Hojka
- & Lu-Ning Liu
-
Article
| Open Access Structure and assembly of cargo Rubisco in two native α-carboxysomes
Structure and assembly of cargo Rubisco in two native α-carboxysomes
Carboxysomes are bacterial microcompartments encapsulating Rubisco and carbonic anhydrase for carbon fixation. Here, authors determine the organization of Rubisco and its interaction with the linker protein CsoS2 within two distant α-carboxysomes.
- Tao Ni
- , Yaqi Sun
- & Peijun Zhang
-
Article
| Open Access Global variation in the fraction of leaf nitrogen allocated to photosynthesis
Global variation in the fraction of leaf nitrogen allocated to photosynthesis
The fraction of leaf nitrogen allocated to RuBisCO indicates differing nitrogen use strategies of plants and varies considerably. Here the authors show that this variation is largely driven by leaf thickness and phosphorus content with light intensity, atmospheric dryness and soil pH also having considerable influence.
- Xiangzhong Luo
- , Trevor F. Keenan
- & Yao Zhang
-
Article
| Open Access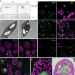 Condensation of Rubisco into a proto-pyrenoid in higher plant chloroplasts
Condensation of Rubisco into a proto-pyrenoid in higher plant chloroplasts
Introducing the pyrenoid-based CO2-concentrating mechanism of green algae into crops could greatly improve photosynthesis. Here, the authors show that expression of the algal linker protein EPYC1 and a plant-algal hybrid Rubisco in Arabidopsis chloroplasts leads to formation of a phase separated algal-like proto-pyrenoid.
- Nicky Atkinson
- , Yuwei Mao
- & Alistair J. McCormick
-
Article
| Open Access The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger
The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger
The microalgal pyrenoid has been reported to behave as a phase-separated liquid compartment. Here the authors demonstrate that the CO2-fixing enzyme Rubisco and the linker protein EPYC1 are necessary and sufficient to bring about a liquid-liquid phase separation that recapitulates the pyrenoid’s liquid-like behavior.
- Tobias Wunder
- , Steven Le Hung Cheng
- & Oliver Mueller-Cajar
-
Article
| Open Access Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts
Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts
Previous efforts to assemble Rubisco within a cyanobacterial carboxysome-derived protein shell in plant chloroplasts to concentrate CO2 have been unsuccessful. Here, Long et al. produce carboxysomes in tobacco chloroplasts that encapsulate the introduced Rubisco and enable autotrophic growth at elevated CO2.
- Benedict M. Long
- , Wei Yih Hee
- & G. Dean Price
-
Article
| Open Access Identification and characterization of multiple rubisco activases in chemoautotrophic bacteria
Identification and characterization of multiple rubisco activases in chemoautotrophic bacteria
The CO2-fixing enzyme rubisco requires motor proteins known as rubisco activases to remove inhibitors bound to its active site. Here the authors describe a new class of rubisco activase present in chemoautotrophic bacteria that belongs to the MoxR family of AAA+ ATPases.
- Yi-Chin Candace Tsai
- , Maria Claribel Lapina
- & Oliver Mueller-Cajar

 An ancient metabolite damage-repair system sustains photosynthesis in plants
An ancient metabolite damage-repair system sustains photosynthesis in plants