Featured
-
-
Article |
Stereospecific alkenylidene homologation of organoboronates by SNV reaction
- Miao Chen
- , Christian D. Knox
- & Guangbin Dong
-
Article |
A deconstruction-reconstruction strategy for pyrimidine diversification
- Benjamin J. H. Uhlenbruck
- , Celena M. Josephitis
- & Andrew McNally
-
Article |
 Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling
Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling
We report on the oxidative cross-coupling of organoboron reagents and amino acids via pyridoxal biocatalysis to produce non-canonical amino acids, uncovering stereoselective, intermolecular free-radical transformations.
- Tian-Ci Wang
- , Binh Khanh Mai
- & Yang Yang
-
Article |
 Copper-catalysed dehydrogenation or lactonization of C(sp3)–H bonds
Copper-catalysed dehydrogenation or lactonization of C(sp3)–H bonds
Use of N-methoxyamides as oxidants enables controllable, redox-neutral, green catalysis of bimodal dehydrogenation/lactonization reactions with methanol as the only by-product.
- Shupeng Zhou
- , Zi-Jun Zhang
- & Jin-Quan Yu
-
Article |
 Couple-close construction of polycyclic rings from diradicals
Couple-close construction of polycyclic rings from diradicals
A couple-close approach used to build semisaturated ring systems from dual radical precursors allows sampling of regions of underexplored chemical space, leading to an annulation that can be used for late-stage functionalization of pharmaceutical scaffolds.
- Alice Long
- , Christian J. Oswood
- & David W. C. MacMillan
-
Article |
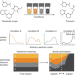 Identifying general reaction conditions by bandit optimization
Identifying general reaction conditions by bandit optimization
Bandit optimization models are used to identify generally applicable conditions by efficient condition sampling and evaluation of experimental feedback.
- Jason Y. Wang
- , Jason M. Stevens
- & Abigail G. Doyle
-
Article |
 Alkene dialkylation by triple radical sorting
Alkene dialkylation by triple radical sorting
We use bimolecular homolytic substitution catalysis to sort an electrophilic radical and a nucleophilic radical across an unactivated alkene, accelerating access to pharmaceutically relevant C(sp3)-rich molecules and defining a mechanistic approach for alkene dialkylation.
- Johnny Z. Wang
- , William L. Lyon
- & David W. C. MacMillan
-
Article
| Open Access Stereodivergent 1,3-difunctionalization of alkenes by charge relocation
Stereodivergent 1,3-difunctionalization of alkenes by charge relocation
We introduce a method for the direct 1,3-difunctionalization of alkenes, based on a concept termed ‘charge relocation’, which enables stereodivergent access to 1,3-difunctionalized products of either syn- or anti-configuration from unactivated alkenes.
- Bogdan R. Brutiu
- , Giulia Iannelli
- & Nuno Maulide
-
Article |
 Carbon-to-nitrogen single-atom transmutation of azaarenes
Carbon-to-nitrogen single-atom transmutation of azaarenes
A new type of transformation converting a heteroaromatic carbon atom into a nitrogen atom, turning quinolines into quinazolines to enable manipulation of molecular properties, is reported.
- Jisoo Woo
- , Colin Stein
- & Mark D. Levin
-
Article |
 Arenium-ion-catalysed halodealkylation of fully alkylated silanes
Arenium-ion-catalysed halodealkylation of fully alkylated silanes
A new method is described that uses arenium-ion-catalysed halodealkylation of silanes with four alkyl groups, typically considered synthetic dead ends, to convert Me4Si and related quaternary silanes into orthogonally substituted (functionalized) silanes.
- Tao He
- , Hendrik F. T. Klare
- & Martin Oestreich
-
Article |
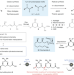 Complex molecule synthesis by electrocatalytic decarboxylative cross-coupling
Complex molecule synthesis by electrocatalytic decarboxylative cross-coupling
We report a radical-based Ni/Ag-electrocatalytic cross-coupling of substituted carboxylic acids, enabling an approach to accessing complex molecular architectures, which relies on a silver additive that forms an active Ag nanoparticle-coated electrode surface along with carefully chosen ligands.
- Benxiang Zhang
- , Jiayan He
- & Phil S. Baran
-
Article |
 Geminal-atom catalysis for cross-coupling
Geminal-atom catalysis for cross-coupling
Heterogeneous geminal-atom catalysts, which pair single-atom sites in specific coordination and spatial proximity, offer a new avenue for the sustainable manufacture of fine chemicals.
- Xiao Hai
- , Yang Zheng
- & Jiong Lu
-
Article |
 Hydrogen-bond-acceptor ligands enable distal C(sp3)–H arylation of free alcohols
Hydrogen-bond-acceptor ligands enable distal C(sp3)–H arylation of free alcohols
Ligands enable alcohol-directed arylation of δ-C(sp3)–H bonds by stabilizing hydroxyl coordination to palladium through charge balance and hydrogen bonding.
- Daniel A. Strassfeld
- , Chia-Yu Chen
- & Jin-Quan Yu
-
Article
| Open Access Light-enabled deracemization of cyclopropanes by Al-salen photocatalysis
Light-enabled deracemization of cyclopropanes by Al-salen photocatalysis
Irradiation of chiral Al-salen complexes with violet light demonstrates efficient deracemization of cyclopropanes, enabling reactivity and enantioselectivity to be regulated simultaneously, negating the requirement for tailored catalyst–substrate recognition motifs.
- Carina Onneken
- , Tobias Morack
- & Ryan Gilmour
-
Article |
 Regioselective aliphatic C–H functionalization using frustrated radical pairs
Regioselective aliphatic C–H functionalization using frustrated radical pairs
Regioselective functionalization of aliphatic carbon–hydrogen bonds is achieved using frustrated radical pairs generated from disilazide donors and an N-oxoammonium acceptor.
- Zhipeng Lu
- , Minsoo Ju
- & Song Lin
-
Article
| Open Access Photocatalytic phosphine-mediated water activation for radical hydrogenation
Photocatalytic phosphine-mediated water activation for radical hydrogenation
Using a photocatalytic phosphine-mediated radical process under mild conditions enables direct hydrogen atom transfer to closed-shell π systems for activation of water.
- Jingjing Zhang
- , Christian Mück-Lichtenfeld
- & Armido Studer
-
Article |
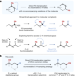 Functional-group translocation of cyano groups by reversible C–H sampling
Functional-group translocation of cyano groups by reversible C–H sampling
Using light-based, reversible C−H sampling catalysis, a cyano functional group can be swapped with a C−H bond in a molecule, providing access to valuable structures that are difficult to obtain by other methods.
- Ken Chen
- , Qingrui Zeng
- & Yan Xu
-
Article |
 General cross-coupling reactions with adaptive dynamic homogeneous catalysis
General cross-coupling reactions with adaptive dynamic homogeneous catalysis
A self-adjustive catalytic system with nickel under visible-light-driven redox reaction conditions provides a general method for carbon–(hetero)atom cross-coupling reactions and is demonstrated for nine different bond-forming reactions.
- Indrajit Ghosh
- , Nikita Shlapakov
- & Burkhard König
-
Article |
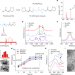 Organic–inorganic covalent–ionic molecules for elastic ceramic plastic
Organic–inorganic covalent–ionic molecules for elastic ceramic plastic
Covalent organic molecules can be combined with ionic inorganic molecules to create a hybrid material demonstrating paradoxical mechanical properties in a bottom-up manner, enabling the manufacture of an ‘elastic ceramic plastic’.
- Weifeng Fang
- , Zhao Mu
- & Zhaoming Liu
-
Article |
 Transannular C–H functionalization of cycloalkane carboxylic acids
Transannular C–H functionalization of cycloalkane carboxylic acids
Quinuclidine-pyridone and sulfonamide-pyridone ligands enable transannular γ-methylene C–H arylation of cycloalkane carboxylic acids with a range of ring sizes, bringing us closer to molecular editing of saturated carbocycles.
- Guowei Kang
- , Daniel A. Strassfeld
- & Jin-Quan Yu
-
Article |
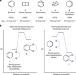 Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives
Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives
The strained C6H6 isomer 1,2,3-cyclohexatriene and its derivatives participate in a host of reaction modes which demonstrate their potential for selective chemical transformations and provide an unconventional entryway to complex scaffolds.
- Andrew V. Kelleghan
- , Ana S. Bulger
- & Neil K. Garg
-
Article |
 General access to cubanes as benzene bioisosteres
General access to cubanes as benzene bioisosteres
The synthesis of 1,3- and 1,2-disubstituted cubanes is achieved using a cyclobutadiene precursor and a photolytic carboxylation reaction, respectively, and copper-catalysed amination, arylation, alkylation and trifluoromethylation reactions have been developed enabling the use of cubanes as bioisosteres of benzenes in drug design.
- Mario P. Wiesenfeldt
- , James A. Rossi-Ashton
- & David W. C. MacMillan
-
Article |
 Copper-catalysed enantioconvergent alkylation of oxygen nucleophiles
Copper-catalysed enantioconvergent alkylation of oxygen nucleophiles
The enantioconvergent alkylation of oxygen nucleophiles is achieved using α-haloamides and a readily available copper catalyst, and the reaction proceeds under mild conditions in the presence of a wide variety of functional groups.
- Caiyou Chen
- & Gregory C. Fu
-
Article |
 Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines
Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines
The chemoselective and enantioconvergent N-alkylation of aliphatic amines, including ammonia, is achieved using chiral tridentate anionic ligands and a copper catalyst; the method shows excellent enantioselectivity and functional-group tolerance.
- Ji-Jun Chen
- , Jia-Heng Fang
- & Xin-Yuan Liu
-
Article
| Open Access Control of stereogenic oxygen in a helically chiral oxonium ion
Control of stereogenic oxygen in a helically chiral oxonium ion
The design, synthesis and characterization of a helically chiral triaryloxonium ion is reported, which is an example of a chiral non-racemic and configurationally stable molecule in which the oxygen atom is the sole stereogenic centre.
- Owen Smith
- , Mihai V. Popescu
- & Martin D. Smith
-
Article |
 Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations
Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations
An electrochemical strategy is described in which the direct carboxylation of pyridines and related N-heteroarenes with CO2 shows divergent site selectivity depending on the type of reactor used.
- Guo-Quan Sun
- , Peng Yu
- & Song Lin
-
Article |
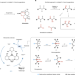 Electrophotocatalytic oxygenation of multiple adjacent C–H bonds
Electrophotocatalytic oxygenation of multiple adjacent C–H bonds
Installation of multiple C–O bonds by concurrent oxygenation of contiguous C–H bonds in a selective fashion is highly desirable, and this is achieved by repeated operation of a potent oxidative catalyst via electrophotocatalysis.
- Tao Shen
- , Yi-Lun Li
- & Tristan H. Lambert
-
Article |
 Discovery of chalcogenides structures and compositions using mixed fluxes
Discovery of chalcogenides structures and compositions using mixed fluxes
A new methodology for the discovery of chalcogenides by tuning the temperature and flux ratios of systems using mixed fluxes is demonstrated, leading to the synthesis of 30 new and unreported compounds or compositions.
- Xiuquan Zhou
- , Venkata Surya Chaitanya Kolluru
- & Mercouri G. Kanatzidis
-
Article |
 Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane
Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane
The potential power of the saturated carbocycle bicyclo[3.1.1]heptane as a beneficial motif for improving the pharmacokinetic and physicochemical properties of drug candidates is demonstrated.
- Nils Frank
- , Jeremy Nugent
- & Edward A. Anderson
-
Article |
 Screening for generality in asymmetric catalysis
Screening for generality in asymmetric catalysis
The analytical workflow outlined in this study allows multiple crude reaction mixtures to be analysed simultaneously, with substantial reductions in method development and analysis time, and maximizes the chances of finding catalytic systems with broad substrate scope.
- Corin C. Wagen
- , Spencer E. McMinn
- & Eric N. Jacobsen
-
Article |
 Photoexcited nitroarenes for the oxidative cleavage of alkenes
Photoexcited nitroarenes for the oxidative cleavage of alkenes
Oxidative cleavage of alkenes is achieved using nitroarenes and light irradiation as an alternative to using ozone to break the carbon–carbon bonds, avoiding the explosive intermediates formed with ozone.
- Alessandro Ruffoni
- , Charlotte Hampton
- & Daniele Leonori
-
Article |
 Molecular editing of aza-arene C–H bonds by distance, geometry and chirality
Molecular editing of aza-arene C–H bonds by distance, geometry and chirality
A unified catalytic remote-directing template strategy enabled precise differentiation of remote and adjacent C6–H and C7–H bonds, and similar C3–H and C7–H bonds of a pharmaceutically relevant bicyclic aza-arene scaffold.
- Zhoulong Fan
- , Xiangyang Chen
- & Jin-Quan Yu
-
Article |
 Cobalt-electrocatalytic HAT for functionalization of unsaturated C–C bonds
Cobalt-electrocatalytic HAT for functionalization of unsaturated C–C bonds
A perspective is given on how an electrocatalytic approach, inspired by decades of energy storage studies, can be used in the context of efficient cobalt-hydride generation with a variety of applications in modern organic synthesis.
- Samer Gnaim
- , Adriano Bauer
- & Phil S. Baran
-
Article |
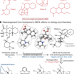 Synthesis and target annotation of the alkaloid GB18
Synthesis and target annotation of the alkaloid GB18
A synthetic route to GB18, an alkaloid from the bark of hallucinogenic Galbulimima sp., is developed, enabling its identification as an antagonist of κ- and μ-opioid receptors.
- Stone Woo
- & Ryan A. Shenvi
-
Article
| Open Access Catalytic synthesis of phenols with nitrous oxide
Catalytic synthesis of phenols with nitrous oxide
A study demonstrates that nitrous oxide can act as the source of O in a catalytic conversion of aryl halides to phenols, releasing N2 as by-product.
- Franck Le Vaillant
- , Ana Mateos Calbet
- & Josep Cornella
-
Article |
 Multifunctional biocatalyst for conjugate reduction and reductive amination
Multifunctional biocatalyst for conjugate reduction and reductive amination
A biocatalytic enzyme originating from bacteria, EneIRED, facilitates amine-activated conjugate alkene reduction followed by reductive amination, efficiently preparing chiral amine diastereomers, which are commonly used in pharmaceuticals and agrochemicals.
- Thomas W. Thorpe
- , James R. Marshall
- & Nicholas J. Turner
-
Article |
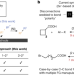 Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling
Ni-electrocatalytic Csp3–Csp3 doubly decarboxylative coupling
An Ni-electrocatalytic system can couple two different carboxylates using doubly decarboxylative cross-coupling, tolerating a range of functional groups, being scalable, used for the synthesis of 32 known compounds and reducing overall step counts by 73%.
- Benxiang Zhang
- , Yang Gao
- & Phil S. Baran
-
Article |
 [18F]Difluorocarbene for positron emission tomography
[18F]Difluorocarbene for positron emission tomography
Carbene chemistry is used to introduce difluoromethyl groups labelled with fluorine-18 into compounds for positron emission tomography imaging, using a reagent designed for high molar activity and versatility.
- Jeroen B. I. Sap
- , Claudio F. Meyer
- & Véronique Gouverneur
-
Article |
 Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer
Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer
A strain-release approach, realized by visible-light-mediated triplet energy transfer catalysis, enabled an intermolecular [2π+2σ]-photocycloaddition.
- Roman Kleinmans
- , Tobias Pinkert
- & Frank Glorius
-
Article |
 Electrochemically driven cross-electrophile coupling of alkyl halides
Electrochemically driven cross-electrophile coupling of alkyl halides
An electrochemical method is used to couple together two alkyl halides, enabling efficient cross-electrophile coupling of a variety of alkyl electrophiles with improved chemoselectivity compared with existing methods.
- Wen Zhang
- , Lingxiang Lu
- & Song Lin
-
Article |
 Synthesis of chiral sulfinate esters by asymmetric condensation
Synthesis of chiral sulfinate esters by asymmetric condensation
A synthetic strategy for the stereoselective preparation of sulfinate esters and related sulfur stereogenic centres via asymmetric condensation expands the drug discovery toolbox for these compounds.
- Xin Zhang
- , Esther Cai Xia Ang
- & Choon-Hong Tan
-
Article |
 Automated iterative Csp3–C bond formation
Automated iterative Csp3–C bond formation
Identification of a hyperstable boronate enables automated lego-like synthesis to access a wider range of three-dimensionally complex small organic molecules rich in Csp3–C bonds.
- Daniel J. Blair
- , Sriyankari Chitti
- & Martin D. Burke
-
Article
| Open Access Tritiation of aryl thianthrenium salts with a molecular palladium catalyst
Tritiation of aryl thianthrenium salts with a molecular palladium catalyst
The isotopic label tritium can be selectively added into aromatic organic compounds by a homogenous hydrogenolysis reaction using aryl thianthrenium salts, tritium gas and a molecular palladium catalyst.
- Da Zhao
- , Roland Petzold
- & Tobias Ritter
-
Article |
 Multicomponent alkene azidoarylation by anion-mediated dual catalysis
Multicomponent alkene azidoarylation by anion-mediated dual catalysis
A streamlined synthesis of β-arylethylamines using two distinct copper catalysts is reported, and an azide anion is proposed to both react to form the product and facilitate catalyst regeneration.
- Ala Bunescu
- , Yusra Abdelhamid
- & Matthew J. Gaunt
-
Article |
 Enantioselective synthesis of ammonium cations
Enantioselective synthesis of ammonium cations
Enantioselective supramolecular recognition allows for the asymmetric synthesis of nitrogen stereocentres, providing chiral ammonium cations in a dynamic crystallization process.
- Mark P. Walsh
- , Joseph M. Phelps
- & Matthew O. Kitching
-
Article |
 Metallaphotoredox-enabled deoxygenative arylation of alcohols
Metallaphotoredox-enabled deoxygenative arylation of alcohols
A metallaphotoredox-based cross-coupling platform is capable of activating a wide range of free alcohols using N-heterocyclic carbene salts, cleaving C–O bonds to form free carbon radicals that are then used to form new C–C bonds.
- Zhe Dong
- & David W. C. MacMillan
-
Article |
 Cleaving arene rings for acyclic alkenylnitrile synthesis
Cleaving arene rings for acyclic alkenylnitrile synthesis
Common aromatic rings, such as anilines, arylboronic acids and aryl halides, can be opened up and converted to alkenyl nitriles through carbon–carbon bond cleavage using a copper catalyst.
- Xu Qiu
- , Yueqian Sang
- & Ning Jiao
-
Article |
 Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity
Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity
In the presence of three ligands and light, two distinct copper catalysts combine to produce enantioenriched secondary amides from racemic alkyl electrophiles and primary amide nucleophiles.
- Caiyou Chen
- , Jonas C. Peters
- & Gregory C. Fu
-
Article |
 Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication
Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication
The synthesis of aziridines—three-membered nitrogen-containing heterocycles—is achieved by a new method involving the electrochemical coupling of alkenes and amines, via a dicationic intermediate.
- Dylan E. Holst
- , Diana J. Wang
- & Zachary K. Wickens
