Featured
-
-
Article |
 X-ray structure of a calcium-activated TMEM16 lipid scramblase
X-ray structure of a calcium-activated TMEM16 lipid scramblase
The authors describe the structure of a Ca2+-activated lipid scramblase which catalyses the passive movement of lipids between the two leaflets of a lipid bilayer; the structure reveals the location of a regulatory calcium-binding site embedded within the membrane and the presence of a hydrophilic membrane-traversing cavity that is exposed to the lipid bilayer, where catalysis is likely to occur.
- Janine D. Brunner
- , Novandy K. Lim
- & Raimund Dutzler
-
Article |
 Structure and insights into the function of a Ca2+-activated Cl− channel
Structure and insights into the function of a Ca2+-activated Cl− channel
The X-ray crystal structure of a eukaryotic Ca2+-activated chloride channel, BEST1, and its function in liposomes are described; the structure shows that Ca2+ binds to the cytosolic region of this pentameric channel and reveals that the pore is approximately 95 Å long with at least 15 distinct anion-binding sites.
- Veronica Kane Dickson
- , Leanne Pedi
- & Stephen B. Long
-
Letter |
 X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors
X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors
This study solved structures of the glutamate-gated chloride channel (GluCl), a Cys-loop receptor from C. elegans, in an apo, closed state and in a lipid-bound state — comparison of these structures with a previously published structure of GluCl in an ivermectin-bound state reveals what conformational changes probably occur as this membrane protein transitions from the closed/resting state towards an open/activated state.
- Thorsten Althoff
- , Ryan E. Hibbs
- & Eric Gouaux
-
Article |
 X-ray structure of the mouse serotonin 5-HT3 receptor
X-ray structure of the mouse serotonin 5-HT3 receptor
The first X-ray crystal structure of the mouse serotonin 5-HT3 receptor, a pentameric ligand-gated ion channel, is similar to those of other Cys-loop receptors — though here electron density for part of the cytoplasmic domain, which is important for trafficking, synaptic localization, and modulation by cytoplasmic proteins, but not visible in previous structures, is also described.
- Ghérici Hassaine
- , Cédric Deluz
- & Hugues Nury
-
Outlook |
 Genetics: Complex expressions
Genetics: Complex expressions
Epilepsy is one of the most common neurological disorders to affect the human brain. Many genetic aspects of the disease have been identified, but mechanisms remains elusive.
- Charvy Narain
-
Article |
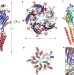 Crystal structure of a human GABAA receptor
Crystal structure of a human GABAA receptor
GABAA receptors are the principal mediators of rapid inhibitor synaptic transmission in the brain, and a decline in GABAA signalling leads to diseases including epilepsy, insomnia, anxiety and autism; here, the first X-ray crystal structure of a human GABAA receptor, the human β3 homopentamer, reveals structural features unique for this receptor class and uncovers the locations of key disease-causing mutations.
- Paul S. Miller
- & A. Radu Aricescu
-
Article |
 TRPV1 structures in distinct conformations reveal activation mechanisms
TRPV1 structures in distinct conformations reveal activation mechanisms
Using a peptide toxin and small vanilloid agonists as pharmacological probes, high-resolution electron cryo-microscopy structures of rat TRPV1–ligand complexes are solved; these structures highlight conformational differences between TRP and voltage-gated ion channels in their active states, and suggest a dual gating mechanism that may account for the ability of members of the TRP channel superfamily to integrate diverse physiological signals.
- Erhu Cao
- , Maofu Liao
- & David Julius
-
Article |
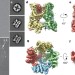 Structure of the TRPV1 ion channel determined by electron cryo-microscopy
Structure of the TRPV1 ion channel determined by electron cryo-microscopy
A high-resolution electron cryo-microscopy structure of the rat transient receptor potential (TRP) channel TRPV1 in its ‘closed’ state is presented; the overall structure of this ion channel is found to share some common features with voltage-gated ion channels, although several unique, TRP-specific features are also characterized.
- Maofu Liao
- , Erhu Cao
- & Yifan Cheng
-
Article |
 Structural basis for Ca2+ selectivity of a voltage-gated calcium channel
Structural basis for Ca2+ selectivity of a voltage-gated calcium channel
X-ray crystal structures of a voltage-gated Na+channel mutated to be highly Ca2+selective provide a framework for understanding the mechanisms of ion selectivity and conductance in vertebrate voltage-gated Ca2+channels.
- Lin Tang
- , Tamer M. Gamal El-Din
- & William A. Catterall
-
Letter |
 Recovery from slow inactivation in K+ channels is controlled by water molecules
Recovery from slow inactivation in K+ channels is controlled by water molecules
A series of long molecular dynamics simulations shows that the K+ channel is sterically locked in the inactive conformation by buried water molecules bound behind the selectivity filter; a kinetic model deduced from the simulations shows how releasing the buried waters can elongate the timescale of the recovery period, and this hypothesis is confirmed using ‘wet’ biophysical experiments.
- Jared Ostmeyer
- , Sudha Chakrapani
- & Benoît Roux
-
Article |
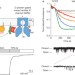 X-ray structure of the mammalian GIRK2–βγ G-protein complex
X-ray structure of the mammalian GIRK2–βγ G-protein complex
An X-ray structure and electrophysiological analysis of mammalian G-protein-gated inward rectifier potassium channel GIRK2 in complex with βγ reveals a pre-open channel structure consistent with channel activation by membrane delimited G-protein subunits.
- Matthew R. Whorton
- & Roderick MacKinnon
-
Article |
 Gating of the TrkH ion channel by its associated RCK protein TrkA
Gating of the TrkH ion channel by its associated RCK protein TrkA
Here it is shown that ion flux through the TrkH–TrkA complex is upregulated by ATP and downregulated by ADP; solving the X-ray crystal structures of the tetrameric TrkA ring in the absence and presence of TrkH suggests a mechanism by which ATP-induced conformational changes in TrkA augment the activity of TrkH.
- Yu Cao
- , Yaping Pan
- & Ming Zhou
-
Article |
 The structure of the KtrAB potassium transporter
The structure of the KtrAB potassium transporter
This study reports the X-ray crystal structure of a Ktr K+ transporter; the structure of this KtrAB complex reveals how the dimeric membrane protein KtrB interacts with the cytosolic octameric KtrA regulatory protein.
- Ricardo S. Vieira-Pires
- , Andras Szollosi
- & João H. Morais-Cabral
-
Letter |
 Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori
Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori
The crystal structure of the inner-membrane urea channel HpUreI from Helicobacter pylori, the causative organism of peptic ulcers, reveals how the channel selectively transports urea across the membrane and buffers the pathogen’s periplasmic pH against the acidic gastric environment.
- David Strugatsky
- , Reginald McNulty
- & Hartmut Luecke
-
Review Article |
 Molecular machines governing exocytosis of synaptic vesicles
Molecular machines governing exocytosis of synaptic vesicles
A brief survey of the molecular mechanisms that give the vesicle cycle in intact synapses its efficiency.
- Reinhard Jahn
- & Dirk Fasshauer
-
Letter |
 Black mamba venom peptides target acid-sensing ion channels to abolish pain
Black mamba venom peptides target acid-sensing ion channels to abolish pain
A new class of peptides, mambalgins, is isolated from the African snake the black mamba, which can abolish pain through inhibition of particular subtypes of acid-sensing ion channels expressed either in central or peripheral neurons.
- Sylvie Diochot
- , Anne Baron
- & Eric Lingueglia
-
Article |
 Structural plasticity and dynamic selectivity of acid-sensing ion channel–spider toxin complexes
Structural plasticity and dynamic selectivity of acid-sensing ion channel–spider toxin complexes
Acid-sensing ion channels (ASICs) are voltage-independent ion channels that participate in a broad range of biological processes, including nociception and mechanosensation; here X-ray crystal structures of the complexes of chicken ASIC1a with psalmotoxin, a peptide toxin from tarantula, indicate that toxin binding triggers an expansion of the extracellular vestibule and stabilization of the open channel pore.
- Isabelle Baconguis
- & Eric Gouaux
-
Letter |
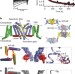 Crystal structure of a voltage-gated sodium channel in two potentially inactivated states
Crystal structure of a voltage-gated sodium channel in two potentially inactivated states
X-ray crystal structures of a bacterial voltage-gated sodium channel in two ‘inactivated’ conformations are reported, revealing several conformational rearrangements that may underlie the electromechanical coupling of voltage sensor movement to inactivation of the pore.
- Jian Payandeh
- , Tamer M. Gamal El-Din
- & William A. Catterall
-
Letter |
 Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel
Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel
The crystal structure of NavRh, a NaChBac orthologue from the marine Rickettsiales sp. HIMB114, defines an ion binding site within the selectivity filter, and reveals several conformational rearrangements that may underlie the electromechanical coupling mechanism.
- Xu Zhang
- , Wenlin Ren
- & Nieng Yan
-
Letter |
 α2δ expression sets presynaptic calcium channel abundance and release probability
α2δ expression sets presynaptic calcium channel abundance and release probability
The voltage-gated calcium channel protein subunit α2δ is shown to control both the abundance of voltage-gated calcium channels and their coupling to the vesicular release of neurotransmitters into the synapse; because the α2δ family is a known target of potent analgesics, this study offers a new link between basic synaptic physiology and pain research in the clinic.
- Michael B. Hoppa
- , Beatrice Lana
- & Timothy A. Ryan
-
Letter |
 Identification and characterization of a bacterial hydrosulphide ion channel
Identification and characterization of a bacterial hydrosulphide ion channel
A channel for the transport of hydrosulphide ions in Clostridium difficile is identified and shown to be polyspecific, being a member of the formate/nitrite transporter family.
- Bryan K. Czyzewski
- & Da-Neng Wang
-
News & Views |
The sensation of stretch
Piezo proteins have been shown to form large ion channels that serve a sensory function in fruitflies. The findings help to explain how Piezos convert mechanical force into biological signals. See Article p.176 & Letter p.209
- Philip A. Gottlieb
- & Frederick Sachs
-
Letter |
 Circadian rhythms govern cardiac repolarization and arrhythmogenesis
Circadian rhythms govern cardiac repolarization and arrhythmogenesis
Circadian rhythmicity of cardiac ion-channel expression and of an index of myocardial repolarization is under the control of Klf15, a clock-dependent oscillator that is required for generating transient outward potassium current, and deficiencies or excesses of which cause loss of rhythmic variation in myocardial and abnormal repolarization, and an enhanced susceptibility to ventricular arrhythmias.
- Darwin Jeyaraj
- , Saptarsi M. Haldar
- & Mukesh K. Jain
-
News & Views |
 Ion channel in the spotlight
Ion channel in the spotlight
When expressed in neurons, channelrhodopsin proteins allow the cells' electrical activity to be controlled by light. The structure of one such protein will guide efforts to make better tools for controlling neurons. See Article p.369
- Oliver P. Ernst
- & Thomas P. Sakmar
-
Letter |
 Gated regulation of CRAC channel ion selectivity by STIM1
Gated regulation of CRAC channel ion selectivity by STIM1
STIM1-mediated gating of CRAC channels occurs through a mechanism in which ion selectivity and gating are closely coupled, and the residue V102 is identified as a candidate for the channel gate.
- Beth A. McNally
- , Agila Somasundaram
- & Murali Prakriya
-
Article |
 Crystal structure of the channelrhodopsin light-gated cation channel
Crystal structure of the channelrhodopsin light-gated cation channel
Channelrhodopsins are light-gated cation channels used in optogenetics; here, the high-resolution crystal structure of a channelrhodopsin from Chlamydomonas reinhardtii is determined.
- Hideaki E. Kato
- , Feng Zhang
- & Osamu Nureki
-
Letter |
 Structure of the carboxy-terminal region of a KCNH channel
Structure of the carboxy-terminal region of a KCNH channel
The function of the KCNH family of potassium channels is critical for the repolarization of the cardiac action potential and the regulation of neuronal excitability; here, the X-ray crystal structure of the cyclic-nuclotide-binding homology domain of the zebrafish ELK channel is reported.
- Tinatin I. Brelidze
- , Anne E. Carlson
- & William N. Zagotta
-
Letter |
 Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel
Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel
The crystal structure of the calcium-bound gating ring of a calcium- and voltage-activated potassium channel shows in detail how the effect of calcium binding on the gating ring produces the conformational change from closed to open.
- Peng Yuan
- , Manuel D. Leonetti
- & Roderick MacKinnon
-
Letter |
 Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila
Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila
Many TRP ion channels respond to more than one category of cue, and how they discriminate between them is largely unknown; the mechanism by which TRPA1 discriminates between sensory stimuli in Drosophila is now determined.
- Kyeongjin Kang
- , Vincent C. Panzano
- & Paul A. Garrity
-
Letter |
 A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain
A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain
- Christopher J. Bohlen
- , Alexander T. Chesler
- & David Julius
-
Letter |
 Aspartate 112 is the selectivity filter of the human voltage-gated proton channel
Aspartate 112 is the selectivity filter of the human voltage-gated proton channel
- Boris Musset
- , Susan M. E. Smith
- & Thomas E. DeCoursey
-
Brief Communications Arising |
 InsP3R channel gating altered by clustering?
InsP3R channel gating altered by clustering?
- Horia Vais
- , J. Kevin Foskett
- & Don-On Daniel Mak
-
Brief Communications Arising |
 Rahman et al. reply
Rahman et al. reply
- Taufiq Rahman
- , Alexander Skupin
- & Colin W. Taylor
-
Letter |
 Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2
Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2
- Scott B. Hansen
- , Xiao Tao
- & Roderick MacKinnon
-
Letter |
 Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats
Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats
- Elena O. Gracheva
- , Julio F. Cordero-Morales
- & David Julius
-
News & Views |
 Peering into the spark of life
Peering into the spark of life
Sodium channels in cell membranes have a crucial role in triggering bioelectrical events that lead to processes such as muscle contraction or hormone release. A crystal structure reveals how one such channel might work. See Article p.353
- Richard Horn
-
Article |
 The crystal structure of a voltage-gated sodium channel
The crystal structure of a voltage-gated sodium channel
- Jian Payandeh
- , Todd Scheuer
- & William A. Catterall
-
Letter |
 A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter
A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter
- Diego De Stefani
- , Anna Raffaello
- & Rosario Rizzuto
-
Letter |
 Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter
Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter
- Joshua M. Baughman
- , Fabiana Perocchi
- & Vamsi K. Mootha
-
Letter |
 Tunable pKa values and the basis of opposite charge selectivities in nicotinic-type receptors
Tunable pKa values and the basis of opposite charge selectivities in nicotinic-type receptors
- Gisela D. Cymes
- & Claudio Grosman
-
Article |
 Principles of activation and permeation in an anion-selective Cys-loop receptor
Principles of activation and permeation in an anion-selective Cys-loop receptor
- Ryan E. Hibbs
- & Eric Gouaux
-
Article |
 Loss-of-function mutations in sodium channel Nav1.7 cause anosmia
Loss-of-function mutations in sodium channel Nav1.7 cause anosmia
- Jan Weiss
- , Martina Pyrski
- & Frank Zufall
-
Letter |
 Progesterone activates the principal Ca2+ channel of human sperm
Progesterone activates the principal Ca2+ channel of human sperm
Progesterone stimulates an increase in Ca2+ levels in human sperm, but the underlying signalling mechanism is poorly understood. Two studies now show that progesterone activates the sperm-specific, pH-sensitive CatSper calcium channel, leading to a rapid influx of Ca2+ ions into the spermatozoa. These results should help to define the physiological role of progesterone and CatSper in sperm, and could lead to the development of new classes of non-hormonal contraceptives.
- Polina V. Lishko
- , Inna L. Botchkina
- & Yuriy Kirichok
-
Letter |
 X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel
X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel
The mechanism of action of general anaesthetics is poorly understood, although there is some evidence that their principal protein targets are pentameric ligand-gated ion channels (pLGICs). Here, the X-ray crystal structures of propofol and desflurane bound to a bacterial homologue of the pLGIC family are solved. The structures reveal a common binding site for these two anaesthetics in the upper part of the transmembrane domain of each protomer.
- Hugues Nury
- , Catherine Van Renterghem
- & Pierre-Jean Corringer
-
Letter |
 A role for mitochondria in NLRP3 inflammasome activation
A role for mitochondria in NLRP3 inflammasome activation
Mitochondrial reactive oxygen species are shown to be required for activation of the NLRP3 inflammasome by various stimuli.
- Rongbin Zhou
- , Amir S. Yazdi
- & Jürg Tschopp
-
Letter |
 Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1
Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1
- Jaime N. Guzman
- , Javier Sanchez-Padilla
- & D. James Surmeier
-
Letter |
 The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule
The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule
Mutations in ryanodine receptors can lead to severe genetic conditions in both cardiac and skeletal muscles. These authors report the X-ray crystal structure of a type 1 ryanodine receptor and pinpoint the exact locations of more than 50 disease-related mutations in the full-length receptor. The disease mutations seem to cause misfolding of an individual domain, to destabilize interactions between the three amino-terminal domains, or to otherwise affect one of the other domain interfaces.
- Ching-Chieh Tung
- , Paolo A. Lobo
- & Filip Van Petegem
-
News & Views |
 A peep through anion channels
A peep through anion channels
The crystal structure of a protein channel provides clues about the mechanisms that control the closure of pores found in the epidermis of plant leaves. Excitingly, the protein channel folds in a way never seen before. See Article p.1074
- Sébastien Thomine
- & Hélène Barbier-Brygoo
-
Article |
 Homologue structure of the SLAC1 anion channel for closing stomata in leaves
Homologue structure of the SLAC1 anion channel for closing stomata in leaves
SLAC1 is a plant ion channel that controls turgor pressure in the guard cells of plant stomata, thereby regulating the exchange of water vapour and photosynthetic gases in response to environmental signals. Here, the X-ray crystal structure of a bacterial homologue of SLAC1 has been solved, and structure-inspired mutagenesis has been used to analyse the conductance properties of the channel. The findings indicate that selectivity among different anions is largely a function of the energetic cost of ion dehydration.
- Yu-hang Chen
- , Lei Hu
- & Wayne A. Hendrickson

 Architecture and conformational switch mechanism of the ryanodine receptor
Architecture and conformational switch mechanism of the ryanodine receptor