Article
|
Open Access
Featured
-
-
Article
| Open Access Long-range allosteric regulation of the human 26S proteasome by 20S proteasome-targeting cancer drugs
Long-range allosteric regulation of the human 26S proteasome by 20S proteasome-targeting cancer drugs
The proteasome regulates several important cellular processes and has been identified as a target for therapeutic interventions. Here the authors map the conformational and energy landscape of the 26S proteasome upon Oprozomib binding and uncover long-range allosteric effects that control the dynamic behaviour of the proteasome.
- David Haselbach
- , Jil Schrader
- & Holger Stark
-
Article
| Open Access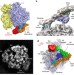 Structure of the quaternary complex between SRP, SR, and translocon bound to the translating ribosome
Structure of the quaternary complex between SRP, SR, and translocon bound to the translating ribosome
Membrane proteins are inserted co-transnationally through the association between ribosome, the signal recognition particle and its receptor, and the membrane-bound translocon. Here the authors present a cryo-EM reconstruction of this quaternary complex in the activated state and propose a model for signal sequence transfer to the translocon.
- Ahmad Jomaa
- , Yu-Hsien Hwang Fu
- & Nenad Ban
-
Article
| Open Access C. elegans chromosomes connect to centrosomes by anchoring into the spindle network
C. elegans chromosomes connect to centrosomes by anchoring into the spindle network
A connection between centrosomes and chromosomes is a key feature of mitotic spindles. Here the authors generate 3D reconstructions of whole mitotic spindles in earlyC. elegansembryos and show that chromosomes are anchored by the entire spindle network and that connections through kinetochore microtubules are few and likely very transient.
- Stefanie Redemann
- , Johannes Baumgart
- & Thomas Müller-Reichert
-
Article
| Open Access Subnanometre-resolution structure of the doublet microtubule reveals new classes of microtubule-associated proteins
Subnanometre-resolution structure of the doublet microtubule reveals new classes of microtubule-associated proteins
Cilia are hair-like appendages involved in cell motility and sensory reception. Here, the authors report a high resolution cryo-EM structure of the microtubule doublet from motile cilia and identify microtubule inner proteins (MIPs) bound to the inner surface of the doublet that appear to stabilize its structure.
- Muneyoshi Ichikawa
- , Dinan Liu
- & Khanh Huy Bui
-
Article
| Open Access Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability
Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability
Japanese encephalitis virus (JEV) is a Flavivirus responsible for thousands of deaths every year for which there are no specific anti-virals. Here, Wang et al. report the cryo-EM structure of mature JEV at near-atomic resolution and identify structural elements that modulate stability and virulence.
- Xiangxi Wang
- , Shi-Hua Li
- & Zihe Rao
-
Article
| Open Access Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains
Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains
Host tropism and cell entry of pathogenic coronaviruses are mediated by their envelope spike (S) proteins. Here the authors present structural analyses of trimeric MERS-CoV and SARS-CoV S proteins in pre-fusion conformation, and reveal two states of the receptor binding domain that suggest new avenues for the generation of neutralizing antibodies.
- Yuan Yuan
- , Duanfang Cao
- & George F. Gao
-
Article
| Open Access Cell-free reconstitution reveals centriole cartwheel assembly mechanisms
Cell-free reconstitution reveals centriole cartwheel assembly mechanisms
The centriole is an organelle composed of rings of SAS-6 proteins that form a cartwheel structure. Here the authors develop a cell-free system to examine core cartwheel assembly ofC. reinhardtiiproteins and discover that CrSAS-6 has autonomous properties that facilitates self-organized stacking of pairs of rings.
- P. Guichard
- , V. Hamel
- & P. Gönczy
-
Article
| Open Access A human antibody against Zika virus crosslinks the E protein to prevent infection
A human antibody against Zika virus crosslinks the E protein to prevent infection
The human monoclonal antibody ZIKV-117 has demonstrated therapeutic potential against Zika while showing no cross-reactivity to other flaviviruses. Here the authors present a cryo-EM structure of the ZIKV strain H/PF/2013 in complex with the ZIKV-117 Fab, shedding light on its neutralization mechanism.
- S. Saif Hasan
- , Andrew Miller
- & Michael G. Rossmann
-
Article
| Open Access Double-stranded RNA virus outer shell assembly by bona fide domain-swapping
Double-stranded RNA virus outer shell assembly by bona fide domain-swapping
Double-shelled bacteriophage φ6 is a well-studied model system used to understand assembly of dsRNA viruses. Here the authors report a near-atomic resolution cryo-EM structure of φ6 and propose a model for the structural transitions occurring in the outer shell during genome packaging.
- Zhaoyang Sun
- , Kamel El Omari
- & Juha T. Huiskonen
-
Article
| Open Access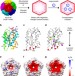 Structure and assembly of scalable porous protein cages
Structure and assembly of scalable porous protein cages
Self-assembling proteins that form capsid-like structures act as molecular containers for diverse cargoes. Here, the authors solve the cryo-EM structures of lumazine synthase shells, and show that supercharged mutants form expanded assemblies, indicating that electrostatics can be exploited to engineer cage architecture.
- Eita Sasaki
- , Daniel Böhringer
- & Donald Hilvert
-
Article
| Open Access Dissecting the molecular organization of the translocon-associated protein complex
Dissecting the molecular organization of the translocon-associated protein complex
The translocon-associated protein complex (TRAP) is a crucial component of the endoplasmic reticulum protein translocon. Here the authors study native translocon structures from human disease patients and algae cells to determine the molecular organization of the TRAP complex.
- Stefan Pfeffer
- , Johanna Dudek
- & Friedrich Förster
-
Article
| Open Access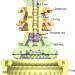 Identical folds used for distinct mechanical functions of the bacterial flagellar rod and hook
Identical folds used for distinct mechanical functions of the bacterial flagellar rod and hook
The bacterial flagellum is a motile organelle that enables bacterial movement. Here the authors explain how the structurally similar flagellum components FlgG and FlgE can give rise to distinct macrostructures—the rod and hook—through subtle differences in domain orientation attributable to a short N-terminal insertion in FlgG.
- Takashi Fujii
- , Takayuki Kato
- & Keiichi Namba
-
Article
| Open Access Structure of actomyosin rigour complex at 5.2 Å resolution and insights into the ATPase cycle mechanism
Structure of actomyosin rigour complex at 5.2 Å resolution and insights into the ATPase cycle mechanism
The cyclic association and dissociation of myosin with actin filament is regulated by ATP binding and hydrolysis cycles. Here the authors report the structure of mammalian skeletal muscle actomyosin rigour complex that provides insights into the ATPase-coupled reaction cycle of actomyosin.
- Takashi Fujii
- & Keiichi Namba
-
Article
| Open Access Structural insights into ribosomal rescue by Dom34 and Hbs1 at near-atomic resolution
Structural insights into ribosomal rescue by Dom34 and Hbs1 at near-atomic resolution
mRNA surveillance is essential to maintain homeostasis in eukaryotes and is activated by mRNAs lacking a stop codon. Here the authors describe a high resolution cryo-EM structure of a nonstop complex that shows how arrested ribosome recognition is achieved during Dom34-mediated mRNA surveillance.
- Tarek Hilal
- , Hiroshi Yamamoto
- & Christian M.T. Spahn
-
Article
| Open Access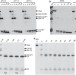 In vitro protease cleavage and computer simulations reveal the HIV-1 capsid maturation pathway
In vitro protease cleavage and computer simulations reveal the HIV-1 capsid maturation pathway
Two competing models—disassembly/reassembly and displacive—have been proposed for how immature spherical HIV virions transform into mature particles with conical cores. Here the authors provide evidence that both disassembly/reassembly and displacive processes occur sequentially during the maturation process.
- Jiying Ning
- , Gonca Erdemci-Tandogan
- & Peijun Zhang
-
Article
| Open Access Atomic structure of the innexin-6 gap junction channel determined by cryo-EM
Atomic structure of the innexin-6 gap junction channel determined by cryo-EM
Gap junctions have critical roles in maintaining homeostasis in multicellular organisms. Here the authors present cryo-EM structures of the C. elegansinnexin-6 gap junction channel, revealing high structural similarity to human connexin 26 despite a different oligomeric number and lack of sequence similarity.
- Atsunori Oshima
- , Kazutoshi Tani
- & Yoshinori Fujiyoshi
-
Article
| Open Access Neutralization mechanism of a highly potent antibody against Zika virus
Neutralization mechanism of a highly potent antibody against Zika virus
There is a pressing need for therapeutic agents against Zika virus (ZIKV). Here the authors present cryoEM structures of a neutralizing antibody (C10) complexed with ZIKV that show C10 preventing structural changes required for virus entry into the cell, suggesting it might be effective in treating Zika infections.
- Shuijun Zhang
- , Victor A. Kostyuchenko
- & Shee-Mei Lok
-
Article
| Open Access Structure of the ribosome post-recycling complex probed by chemical cross-linking and mass spectrometry
Structure of the ribosome post-recycling complex probed by chemical cross-linking and mass spectrometry
Ribosome recycling orchestrated by ABCE1 connects translation termination and mRNA surveillance mechanisms with re-initiation. Using a cross-linking and mass spectrometry approach, Kiosze-Becker et al. provide new information on the large conformational rearrangements that occur during ribosome recycling.
- Kristin Kiosze-Becker
- , Alessandro Ori
- & Robert Tampé
-
Article
| Open Access Cryo-EM study of start codon selection during archaeal translation initiation
Cryo-EM study of start codon selection during archaeal translation initiation
Initiation factor eIF2, common to eukaryotes and archaea, is a central actor in translation initiation. Here the authors describe two cryo-EM structures of archaeal 30S initiation complexes that provide a novel view of the central role that e/aIF2 plays in start codon selection.
- Pierre-Damien Coureux
- , Christine Lazennec-Schurdevin
- & Yves Mechulam
-
Article
| Open Access Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions
Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions
The bacterial flagellar hook is made up of many copies of the protein FlgE. Here, the authors report the full structure of the hook from Campylobacter jejuni and show that its overall structure is different from that of the previously published filament.
- Hideyuki Matsunami
- , Clive S. Barker
- & Fadel A. Samatey
-
Article
| Open Access Structures and stabilization of kinetoplastid-specific split rRNAs revealed by comparing leishmanial and human ribosomes
Structures and stabilization of kinetoplastid-specific split rRNAs revealed by comparing leishmanial and human ribosomes
Leishmania donovani is a protozoan parasite that can cause fatal infections in humans. Here the authors present a high resolution cryoEM structure of the L. donovani80S ribosome and compare it to its human counterpart to provide insight into the basis for drug selectivity towards this eukaryotic parasite.
- Xing Zhang
- , Mason Lai
- & Z. Hong Zhou
-
Article
| Open Access Structure of the Neisseria meningitidis Type IV pilus
Structure of the Neisseria meningitidis Type IV pilus
Type IV pili are present on a wide range of bacterial pathogens and mediate diverse functions. Here the authors report a high resolution crystal structure of the pilin subunit PilE, and a cryoEM reconstruction of the Type IV pilus filament from N. meningitidisthat offer insight into pilus assembly and functions.
- Subramania Kolappan
- , Mathieu Coureuil
- & Lisa Craig
-
Article
| Open Access Lateral opening in the intact β-barrel assembly machinery captured by cryo-EM
Lateral opening in the intact β-barrel assembly machinery captured by cryo-EM
The β-barrel assembly machinery (BAM complex) is a key mediator of outer membrane protein biogenesis in Gram-negative bacteria. Here the authors report a cryo-EM structure of the intact BAM complex that suggests that lateral gate opening is a necessary part of the BAM functional cycle.
- Matthew G. Iadanza
- , Anna J. Higgins
- & Neil A. Ranson
-
Article
| Open Access Structure–function insights reveal the human ribosome as a cancer target for antibiotics
Structure–function insights reveal the human ribosome as a cancer target for antibiotics
The ribosome of bacteria and other unicellular pathogens is a common target for antibiotic drugs. Here the authors determine a structure of the human ribosome bound to the translation inhibitor cycloheximide, and provide evidence that targeting the ribosome is a promising avenue for cancer therapy.
- Alexander G. Myasnikov
- , S. Kundhavai Natchiar
- & Bruno P. Klaholz
-
Article
| Open Access Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ
Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ
MS2 is a single-stranded RNA bacteriophage that infects its host via adsorption to bacterial pili. Here the authors visualize the MS2 virion with asymmetric cryo-EM reconstruction, revealing that the genome of MS2 adopts a specific structure of asymmetrically distributed stem-loops connected to the capsid.
- Roman I Koning
- , Josue Gomez-Blanco
- & Abraham J. Koster
-
Article
| Open Access Ribosomal 18S rRNA base pairs with mRNA during eukaryotic translation initiation
Ribosomal 18S rRNA base pairs with mRNA during eukaryotic translation initiation
Prokaryotic translation initiation involves mRNA-ribosomal RNA base pairing interactions. Here, the authors provide evidence for a similar base pairing interactions occurring between the human h4 mRNA and helix 16 of the small subunit rRNA to position the correct AUG codon in the decoding site.
- Franck Martin
- , Jean-François Ménétret
- & Gilbert Eriani
-
Article
| Open Access Topology and structure of an engineered human cohesin complex bound to Pds5B
Topology and structure of an engineered human cohesin complex bound to Pds5B
Cohesin is a ring-shaped protein complex that structures chromatin and mediates sister chromatid cohesion. Here the authors rigidify cohesin using engineered Smc1 and Smc3 and generated 3D models showing how Pds5B forms an integral part of the cohesin ring.
- Michael T. Hons
- , Pim J. Huis in ‘t Veld
- & Jan-Michael Peters
-
Article
| Open Access Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process
Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process
Aerolysin is a secreted bacterial pore forming toxin that inserts into the host plasma membrane, potentially leading to cell death. Here the authors present Cryo-EM structures of aerolysin arrested at different stages of the pore formation process that provide insight into the conformational changes that allow pore formation.
- Ioan Iacovache
- , Sacha De Carlo
- & Benoît Zuber
-
Article
| Open Access A combined cryo-EM and molecular dynamics approach reveals the mechanism of ErmBL-mediated translation arrest
A combined cryo-EM and molecular dynamics approach reveals the mechanism of ErmBL-mediated translation arrest
When the antibiotic erythromycin is bound to the ribosomal exit tunnel, ErmBL peptide translation stalls and allows translation of the downstream methyltransferase ErmB. Here the authors combine cryo-EM and molecular dynamics simulations to identify the underlying basis for the inhibition of peptide bond formation that results in ribosome stalling.
- Stefan Arenz
- , Lars V. Bock
- & Daniel N. Wilson
-
Article
| Open Access Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein
Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein
Lysenin is member of the aerolysin family of small ß-barrel pore-forming toxins that include virulence factors from several human and animal pathogens. Here the authors determine the structure of the lysenin pore by single particle cryo- EM and propose a conserved pore formation mechanism for the aerolysin protein family.
- Monika Bokori-Brown
- , Thomas G. Martin
- & Christos G. Savva
-
Article
| Open Access Three-dimensional structural dynamics and fluctuations of DNA-nanogold conjugates by individual-particle electron tomography
Three-dimensional structural dynamics and fluctuations of DNA-nanogold conjugates by individual-particle electron tomography
The control of DNA-mediated assembly depends on a precise understanding of the three-dimensional structure of DNA-nanocrystal-hybridized building blocks. Here, the authors use cryo-electron microscopy and negative-staining techniques to investigate the morphology of DNA-nanogold conjugates.
- Lei Zhang
- , Dongsheng Lei
- & Gang Ren
-
Article
| Open Access Structure of the full-length TRPV2 channel by cryo-EM
Structure of the full-length TRPV2 channel by cryo-EM
Transient receptor potential (TRP) proteins are Ca2+-permeable cation channels activated by a range of chemical and physical stimuli. Here the authors describe a cryo-EM structure of the full-length TRPV2 channel that provides insight into the regulation of the TRPV subfamily of channels.
- Kevin W. Huynh
- , Matthew R. Cohen
- & Vera Y. Moiseenkova-Bell
-
Article
| Open Access Damage-free vibrational spectroscopy of biological materials in the electron microscope
Damage-free vibrational spectroscopy of biological materials in the electron microscope
Use of electron microscopy to determine morphology, or find where functionally significant biomolecules are located with high spatial resolution is of great interest. Here, Rez, Cohen et al. use aloof electron beam vibrational spectroscopy to probe different bonds in biological samples with no significant radiation damage.
- Peter Rez
- , Toshihiro Aoki
- & Hagai Cohen
-
Article
| Open Access Cryo-EM structures of the eukaryotic replicative helicase bound to a translocation substrate
Cryo-EM structures of the eukaryotic replicative helicase bound to a translocation substrate
The Cdc45-MCM-GINS (CMG) helicase unwinds DNA during replication, a process that requires the ATPase-dependent activity of the MCM complex. Using cryo-EM reconstructions of the CMG complex in different conformations, the authors propose a model where the N-terminal and AAA+ domains of MCM work in concert to translocate along DNA.
- Ferdos Abid Ali
- , Ludovic Renault
- & Alessandro Costa
-
Article
| Open Access Volta phase plate cryo-EM of the small protein complex Prx3
Volta phase plate cryo-EM of the small protein complex Prx3
Although the resolution achievable with cryo-EM can now rival crystallography, its application to small protein assemblies remains challenging. Here the authors demonstrate the use of the Volta phase plate for single particle analysis and its potential for the study of small specimens.
- Maryam Khoshouei
- , Mazdak Radjainia
- & Radostin Danev
-
Article
| Open Access The architecture of the Schizosaccharomyces pombe CCR4-NOT complex
The architecture of the Schizosaccharomyces pombe CCR4-NOT complex
CCR4-NOT is a protein complex involved in a variety of important genetic processes. Here, the authors report the mid-resolution structure of this complex, and model the positions and contacts between the subunits, providing structural support for the previously reported functions of the complex.
- Marta Ukleja
- , Jorge Cuellar
- & Jose M. Valpuesta
-
Article
| Open Access Structure of a bacterial type III secretion system in contact with a host membrane in situ
Structure of a bacterial type III secretion system in contact with a host membrane in situ
Bacterial type III secretion systems (T3SSs) inject virulence effector proteins into eukaryotic cells and are activated by host membrane contact. Here the authors report the in situ structure of the Chlamydia trachomatisT3SS in the presence or absence of host membrane, and observe compaction of the basal body embedded in the bacterial envelope.
- Andrea Nans
- , Mikhail Kudryashev
- & Richard D. Hayward
-
Article
| Open Access Direct visualization of dispersed lipid bicontinuous cubic phases by cryo-electron tomography
Direct visualization of dispersed lipid bicontinuous cubic phases by cryo-electron tomography
Dispersed lipid self-assembly can form various types of particles, including cubosomes, which are useful for drug delivery. Here, Demurtas et al. visualize their three-dimensional structure, showing two continuous water channels separated by lipid bilayers and the mechanism of particle stabilization.
- Davide Demurtas
- , Paul Guichard
- & Laurent Sagalowicz
-
Article
| Open Access Localized reconstruction of subunits from electron cryomicroscopy images of macromolecular complexes
Localized reconstruction of subunits from electron cryomicroscopy images of macromolecular complexes
Electron cryomicroscopy can allow the elucidation of macromolecular structures; however, mismatches in symmetry between different components limit the attainable resolution. Here, the authors set out a computational method for extracting and retaining information from such components.
- Serban L. Ilca
- , Abhay Kotecha
- & Juha T. Huiskonen
-
Article
| Open Access Antibodies to a conformational epitope on gp41 neutralize HIV-1 by destabilizing the Env spike
Antibodies to a conformational epitope on gp41 neutralize HIV-1 by destabilizing the Env spike
The envelope glycoprotein (Env) trimer is the only antigenic target for broadly neutralizing antibodies on the surface of the HIV-1 virus. Here the authors show that two related monoclonal antibodies bind between gp41 protomers and neutralize HIV-1 by accelerating Env trimer decay.
- Jeong Hyun Lee
- , Daniel P. Leaman
- & Andrew B. Ward
-
Article
| Open Access Large-volume en-bloc staining for electron microscopy-based connectomics
Large-volume en-bloc staining for electron microscopy-based connectomics
Large-scale dense reconstruction of neuronal circuits (or connectomics) requires methods for large-volume dense en-blocelectron microscopy (EM) staining. Here the authors develop a protocol for staining tissue blocks from mouse neocortex sized at least 1 mm in diameter, enabling correlated functional and structural circuit analyses.
- Yunfeng Hua
- , Philip Laserstein
- & Moritz Helmstaedter
-
Article
| Open Access Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-Å resolution
Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-Å resolution
The Hepatitis C virus (HCV) relies on an internal ribosome entry site (IRES) for translation of all the proteins encoded by its single-stranded RNA genome. Here the authors present a near-atomic cryo-EM structure of the HCV IRES bound to the human ribosome, shedding light on the initiation mechanism of HCV's and related IRESs.
- Nick Quade
- , Daniel Boehringer
- & Nenad Ban
-
Article
| Open Access Cryo-EM structure of the bacteriophage T4 portal protein assembly at near-atomic resolution
Cryo-EM structure of the bacteriophage T4 portal protein assembly at near-atomic resolution
Tailed bacteriophages translocate the genome into and out of the capsid through a portal protein assembly located between the phage s head and tail. Here Sun et al. provide a cryo-EM structure of the bacteriophage T4 portal protein assembly, suggesting the functions and evolution of the portal structure.
- Lei Sun
- , Xinzheng Zhang
- & Michael G. Rossmann
-
Article
| Open Access Cryo-EM reveals the conformation of a substrate analogue in the human 20S proteasome core
Cryo-EM reveals the conformation of a substrate analogue in the human 20S proteasome core
The proteasome is a highly regulated complex fundamental for cell homeostasis and a target for cancer therapy. Here the authors use cryo-EM and single-particle analysis to obtain a detailed map of the interactions between each active sites of the core 20S proteasome and the irreversible inhibitor AdaAhx3L3VS.
- Paula C.A. da Fonseca
- & Edward P. Morris
-
Article
| Open Access Graphene-enabled electron microscopy and correlated super-resolution microscopy of wet cells
Graphene-enabled electron microscopy and correlated super-resolution microscopy of wet cells
Preparing biological material for electron microscopy (EM) involves harsh processing steps that can poorly preserve cellular ultrastructure. Here the authors apply a single layer of graphene onto wet cells to enable direct EM using low voltage, and correlate actin filaments and mitochondria using super-resolution microscopy.
- Michal Wojcik
- , Margaret Hauser
- & Ke Xu
-
Article |
 Organization of the mitochondrial translation machinery studied in situ by cryoelectron tomography
Organization of the mitochondrial translation machinery studied in situ by cryoelectron tomography
While the cytosolic translation machinery is well characterized, the mitochondrial translation system remains largely elusive. Using cryo-electron tomography, Pfeffer et al. describe the ordered organization of mitochondrial polysomes in which each ribosome is tethered to the inner membrane by two defined contacts on the large subunit in situ.
- Stefan Pfeffer
- , Michael W. Woellhaf
- & Friedrich Förster
-
Article |
 Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia
Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia
Our current understanding of cilia biology and ciliary diseases is incomplete, in part because cilia are hard to visualize. Here, the authors use cryo-electron tomography to image the structure of human cilia with high resolution and uncover the elusive ciliary defects in Primary Ciliary Dyskinesia patients.
- Jianfeng Lin
- , Weining Yin
- & Daniela Nicastro
-
Article |
 High-resolution structure of the Shigella type-III secretion needle by solid-state NMR and cryo-electron microscopy
High-resolution structure of the Shigella type-III secretion needle by solid-state NMR and cryo-electron microscopy
Solving structures of large protein complexes remains a significant challenge for structural biologists. Demers et al. determine the atomic structure of a Shigellatype-III secretion system using a Rosetta-based modelling strategy that draws on both solid-state NMR and cryo-electron microscopy data sets.
- Jean-Philippe Demers
- , Birgit Habenstein
- & Nikolaos G. Sgourakis
-
Article
| Open Access An atomic model of brome mosaic virus using direct electron detection and real-space optimization
An atomic model of brome mosaic virus using direct electron detection and real-space optimization
Recent developments in cryo-electron microscopy have enabled structure determination of large protein complexes at almost atomic resolution. Wang et al.combine some of these technologies into an effective workflow, and demonstrate the protocol by solving the atomic structure of an icosahedral RNA virus.
- Zhao Wang
- , Corey F. Hryc
- & Wah Chiu

 Architecture of the RNA polymerase II-Paf1C-TFIIS transcription elongation complex
Architecture of the RNA polymerase II-Paf1C-TFIIS transcription elongation complex