Featured
-
-
Letter |
 The BC component of ABC toxins is an RHS-repeat-containing protein encapsulation device
The BC component of ABC toxins is an RHS-repeat-containing protein encapsulation device
The crystal structure of the complex formed by the B and C toxin complex proteins is reported, revealing how toxin complexes are processed and protected; the proteins assemble to form a large hollow structure that sequesters the cytotoxic portion of the C protein, and a β-propeller domain mediates attachment to the A protein in the native ABC complex.
- Jason N. Busby
- , Santosh Panjikar
- & J. Shaun Lott
-
Letter |
 Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics
Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics
The structure of the HIV-1 capsid is analysed by cryo-electron microscopy and cryo-electron tomography, allowing presentation of an all-atom molecular dynamics model of the entire capsid.
- Gongpu Zhao
- , Juan R. Perilla
- & Peijun Zhang
-
Letter |
 Reconfiguration of the proteasome during chaperone-mediated assembly
Reconfiguration of the proteasome during chaperone-mediated assembly
The proteasome degrades ubiquitin-conjugated substrates; here, structural and functional insights from studies in yeast reveal that it is reconfigured during chaperone-mediated assembly.
- Soyeon Park
- , Xueming Li
- & Daniel Finley
-
Article |
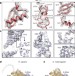 Structures of the human and Drosophila 80S ribosome
Structures of the human and Drosophila 80S ribosome
High-resolution cryo-EM density maps are used to present the structures of Drosophila and human 80S ribosomes in complex with eEF2, E-site transfer RNA and Stm1-like proteins, and reveal the presence of two additional structural layers in the ribosomes of metazoan eukaryotes.
- Andreas M. Anger
- , Jean-Paul Armache
- & Roland Beckmann
-
Article |
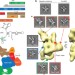 The architecture of Tetrahymena telomerase holoenzyme
The architecture of Tetrahymena telomerase holoenzyme
The long-awaited structure of a telomerase holoenzyme, from Tetrahymena, has been obtained by electron microscopy; affinity labelling of subunits and modelling with NMR and crystal structures of various components allowed the identification of the catalytic core and subunit interactions, and the functional role of the subunits in telomerase processivity was enabled by performing the first reconstitution of the holoenzyme in vitro.
- Jiansen Jiang
- , Edward J. Miracco
- & Juli Feigon
-
Letter |
 A syringe-like injection mechanism in Photorhabdus luminescens toxins
A syringe-like injection mechanism in Photorhabdus luminescens toxins
The TcA component of Photorhabdus luminescens ABC-type toxin complexes forms a transmembrane pore and injects TcC, the functional component of the toxin, into the target cell by means of a syringe-like mechanism.
- Christos Gatsogiannis
- , Alexander E. Lang
- & Stefan Raunser
-
Article |
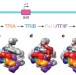 Structural visualization of key steps in human transcription initiation
Structural visualization of key steps in human transcription initiation
Cryo-electron microscopy structures of key intermediates during the sequential assembly of the pre-initiation complex are presented; structures of the closed and open-promoter complexes allow insights into the process of promoter melting.
- Yuan He
- , Jie Fang
- & Eva Nogales
-
Letter |
 The architecture of human general transcription factor TFIID core complex
The architecture of human general transcription factor TFIID core complex
The structures of three distinct human transcription factor IID (TFIID) protein assemblies are solved using cryo-electron microscopy; by incorporating TAF8 and TAF10, the key structural changes that remodel TFIID during assembly are determined, particularly the transition from a symmetric core-TFIID to an asymmetric holo-complex.
- Christoph Bieniossek
- , Gabor Papai
- & Imre Berger
-
Letter |
 Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy
Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy
A hybrid cryo-electron microscopy/tomography approach is used to solve the structure of the immature Mason–Pfizer monkey virus Gag lattice at a resolution of 8 Å, allowing the derivation of a model for the structure of retroviral capsid in the immature Gag shell.
- Tanmay A. M. Bharat
- , Norman E. Davey
- & John A. G. Briggs
-
Letter |
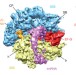 The complex of tmRNA–SmpB and EF-G on translocating ribosomes
The complex of tmRNA–SmpB and EF-G on translocating ribosomes
Stalled bacterial ribosomes can be rescued by interaction with SmpB protein and a highly structured transfer-messenger RNA, and a cryo-electron microscopy map of this complex now shows how EF-G-dependent translocation of this non-canonical ligand is facilitated by conformational changes in the ribosome and the transfer-messenger RNA.
- David J. F. Ramrath
- , Hiroshi Yamamoto
- & Christian M. T. Spahn
-
Article |
 Type VI secretion requires a dynamic contractile phage tail-like structure
Type VI secretion requires a dynamic contractile phage tail-like structure
Microscopy reveals the dynamics of the type VI secretion system of Vibrio cholerae and its structural and functional resemblance to the contractile tail sheath of bacteriophages.
- M. Basler
- , M. Pilhofer
- & J. J. Mekalanos
-
Article |
 Structure and mechanism of the chromatin remodelling factor ISW1a
Structure and mechanism of the chromatin remodelling factor ISW1a
- Kazuhiro Yamada
- , Timothy D. Frouws
- & Timothy J. Richmond
-
Research Highlights |
Glimpses of crystal growth
-
Letter |
 Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
During translation, tRNAs enter the ribosome and then move sequentially through three sites, known as A, P and E, as they transfer their attached amino acids onto the growing peptide chain. How the ribosome facilitates tRNA translocation between the sites remains largely unknown. Now a study uses multiparticle cryoelectron microscopy of a ribosome bound to the translation elongation factor, EF-G, to get information about tRNA movement. It identifies two new substates and sees that translocation is linked to unratcheting of the 30S ribosomal subunit.
- Andreas H. Ratje
- , Justus Loerke
- & Christian M. T. Spahn
-
News |
 Electron microscopy gets twisted
Electron microscopy gets twisted
Spiralling electron beams have the potential to measure and manipulate the properties of single atoms.
- Zeeya Merali
-
Article |
 Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
During protein synthesis within the ribosome, transfer RNAs (tRNAs) move sequentially through different sites as their attached amino acids are transferred onto the growing protein chain. Large conformational movements accompany this process. Here, a staggering 1.9 million electron cryomicroscopy images of the ribosome have been processed to visualize these changes. The results reveal that the ribosome functions as a Brownian machine that couples spontaneous changes driven by thermal energy to directed movement.
- Niels Fischer
- , Andrey L. Konevega
- & Holger Stark
-
News & Views |
 Translocation in slow motion
Translocation in slow motion
Time-resolved electron microscopy can capture structural changes in active macromolecular complexes, but detailed imaging is essential. The dynamics of one step in protein synthesis has been deduced from two million images.
- Måns Ehrenberg
-
News |
 Japan rolls out elite science funds
Japan rolls out elite science funds
FIRST scheme targets large grants to world-leading researchers.
- David Cyranoski

 Visualizing virus assembly intermediates inside marine cyanobacteria
Visualizing virus assembly intermediates inside marine cyanobacteria