News & Views |
Featured
-
-
Letter |
 Geometry-induced electrostatic trapping of nanometric objects in a fluid
Geometry-induced electrostatic trapping of nanometric objects in a fluid
Many fields would benefit from a simple and efficient method of trapping single particles, but this is extremely difficult when dealing with nanometre-sized objects in solution. These authors show that grooves and pockets etched into fluidic channels that acquire a charge on exposure act as highly effective electrostatic traps. With further optimization, this trapping concept could allow contact-free confinement of single proteins and nanoparticles, their sorting and fractionation, or assembly into high-density arrays.
- Madhavi Krishnan
- , Nassiredin Mojarad
- & Vahid Sandoghdar
-
Research Highlights |
Biophysics: DNA replication control
-
Letter |
 Two enzymes bound to one transfer RNA assume alternative conformations for consecutive reactions
Two enzymes bound to one transfer RNA assume alternative conformations for consecutive reactions
In most bacteria and all archaea, glutamyl-tRNA synthetase (GluRS) glutamylates both tRNAGlu and tRNAGln; Glu-tRNAGln is then converted to Gln-tRNAGln by an amidotransferase. Here the structure is reported of a bacterial complex containing tRNAGln, GluRS and the amidotransferase GatCAB. The structure provides an explanation for how the enzymes work consecutively: only one can assume a productive state at any time. There also seems to be an intermediary state in which neither enzyme is productive.
- Takuhiro Ito
- & Shigeyuki Yokoyama
-
Letter |
 Structure of a fucose transporter in an outward-open conformation
Structure of a fucose transporter in an outward-open conformation
In Escherichia coli, the uptake of L-fucose, an important source of carbon for microorganisms, is mediated by a proton symporter from the major facilitator superfamily (MFS). These authors report the first X-ray crystal structure of the outward-open conformation of an MFS proton transporter, FucP. Building on previous work, they develop a working model for how the substrate is recognized by the transporter and how the protein mediates L-fucose/proton symport.
- Shangyu Dang
- , Linfeng Sun
- & Nieng Yan
-
Letter |
 Conical intersection dynamics of the primary photoisomerization event in vision
Conical intersection dynamics of the primary photoisomerization event in vision
Chemical reactions are usually described in terms of the movement of nuclei between the potential energy surfaces of ground and excited electronic states. Crossings known as conical intersections permit efficient transitions between the surfaces. It is shown here that ultrafast optical spectroscopy, with sub-20-fs time resolution and spectral coverage from the visible to the near-infrared, can map the isomerization of rhodopsin with sufficient resolution to shown that a conical intersection is important in this crucial event in vision.
- Dario Polli
- , Piero Altoè
- & Giulio Cerullo
-
News & Views |
 Seaming is believing
Seaming is believing
Do excited molecules relaxing to their ground state pass through a 'seam' connecting the potential energy profiles of the states? Experimental data suggest the answer to this long-standing question is 'yes'. See Letter p. 440
- Todd J. Martinez
-
News & Views |
 A new phase for X-ray imaging
A new phase for X-ray imaging
A fine marriage between two approaches to X-ray microscopy — computed tomography and ptychographic imaging — delivers high-resolution, three-dimensional images of samples without the need for lenses. See Letter p. 436
- Henry N. Chapman
-
Letter |
 Structure of a cation-bound multidrug and toxic compound extrusion transporter
Structure of a cation-bound multidrug and toxic compound extrusion transporter
Transporter proteins from the MATE (multidrug and toxic compound extrusion) family are involved in metabolite transport in plants, and in multiple-drug resistance in bacteria and mammals. Here, the X-ray crystal structure of a MATE transporter from Vibrio cholerae is reported. The structure is in an outward-facing conformation, and reveals a cation-binding site near to residues previously deemed essential for transport.
- Xiao He
- , Paul Szewczyk
- & Geoffrey Chang
-
Letter |
 Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows
Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows
In the one-cell Caenorhabditis elegans embryo, anteroposterior polarization is facilitated by large-scale flow of the actomyosin cortex, which segregates cortical polarity proteins into anterior and posterior domains. The underlying forces and physical principles behind long-range flow are unclear. Here, a new method is described by which to measure cortical tension. The results identify two prerequisites for large-scale cortical flow: a gradient in actomyosin contractility and a sufficiently large viscosity of the cortex.
- Mirjam Mayer
- , Martin Depken
- & Stephan W. Grill
-
Letter |
 In vivo imaging of labelled endogenous β-actin mRNA during nucleocytoplasmic transport
In vivo imaging of labelled endogenous β-actin mRNA during nucleocytoplasmic transport
Newly synthesized messenger RNA is exported from the nucleus through nuclear pores. Here, a new imaging and tracking method has been developed to study the kinetics of mRNA export, with 20-ms time-precision and 26-nm spatial precision. A three-step model for export is presented, comprising docking, transport and release. Notably, mRNAs can move bi-directionally through the pore complex.
- David Grünwald
- & Robert H. Singer
-
Letter |
 Direct visualization of secondary structures of F-actin by electron cryomicroscopy
Direct visualization of secondary structures of F-actin by electron cryomicroscopy
The formation of filamentous F-actin, through polymerization of globular G-actin, is essential for processes such as cell motility and muscle contraction. These authors report the structure of F-actin as visualized by electron cryomicroscopy, and build a complete atomic model of F-actin. This new structure will improve our understanding of the mechanism of actin assembly and disassembly.
- Takashi Fujii
- , Atsuko H. Iwane
- & Keiichi Namba
-
Letter |
 Structural basis of Na+-independent and cooperative substrate/product antiport in CaiT
Structural basis of Na+-independent and cooperative substrate/product antiport in CaiT
Transport of solutes across biological membranes is carried out by specialized secondary transport proteins in the lipid bilayer. These authors report structures of the sodium-independent carnitine/butyrobetaine antiporter CaiT from two microorganisms. The three-dimensional architecture of CaiT resembles that of the Na+-dependent transporters LeuT and BetP, but in CaiT a methionine sulphur takes the place of the Na+ ion to coordinate the substrate in the central transport site, enabling Na+-independent transport to occur.
- Sabrina Schulze
- , Stefan Köster
- & Werner Kühlbrandt
-
-
Article |
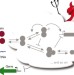 Fundamental limits on the suppression of molecular fluctuations
Fundamental limits on the suppression of molecular fluctuations
Biological systems avoid molecular noise using feedback loops controlling RNA or protein synthesis, but these reactions rely on the stochastic birth and death of molecules. These authors use control and information theory to show that making a genetic network twice as accurate takes 16 times more signalling steps. Nature must therefore call on brute-force solutions to maintain accuracy, and hence does so only when noise suppression is absolutely vital.
- Ioannis Lestas
- , Glenn Vinnicombe
- & Johan Paulsson
-
Letter |
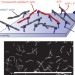 Polar patterns of driven filaments
Polar patterns of driven filaments
Collective motion is a ubiquitous self-organization phenomenon that can be observed in systems ranging from flocks of animals to the cytoskeleton. Similarities between these systems suggest that there are universal underlying principles. This idea can be tested with 'active' or 'driven' fluids, but so far such systems have offered limited parameter control. Here, an active fluid is studied that contains only a few components — actin filaments and molecular motors — allowing the control of all relevant system parameters.
- Volker Schaller
- , Christoph Weber
- & Andreas R. Bausch
-
Letter |
 The structure of (CENP-A–H4)2 reveals physical features that mark centromeres
The structure of (CENP-A–H4)2 reveals physical features that mark centromeres
The centromeres of chromosomes are specified epigenetically, and the histone H3 variant CENP-A is assembled into the chromatin of all active centromeres. Here, the crystal structure of CENP-A in a tetrameric complex with histone H4 reveals the physical features of centromeric chromatin. CENP-A seems to mark the centromere by altering nucleosome structure from within its folded histone core.
- Nikolina Sekulic
- , Emily A. Bassett
- & Ben E. Black
-
Letter |
 A mechanically stabilized receptor–ligand flex-bond important in the vasculature
A mechanically stabilized receptor–ligand flex-bond important in the vasculature
Here, a new type of behaviour of receptor–ligand bonds has been identified, by using a new method that links receptor and ligand in a single molecule to measure binding and unbinding. The binding of von Willebrand factor to the glycoprotein Ib α subunit on the surface of platelets is important for coagulation. This receptor–ligand bond is now shown to have two distinct states, one seen at low force and a second that has greater force resistance. This has implications for how increased blood flow activates platelet plug formation.
- Jongseong Kim
- , Cheng-Zhong Zhang
- & Timothy A. Springer
-
Letter |
 Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase
Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase
The repair enzyme (6–4) photolyase uses light energy to cleave the ultraviolet-induced bond between pyrimidine dimers. These authors use ultrafast spectroscopy to examine the detailed electron and proton movements during the catalytic photocycle. Histidine 364 is identified as the crucial residue involved in the rate-limiting step.
- Jiang Li
- , Zheyun Liu
- & Dongping Zhong
-
Letter |
 Structure of the LexA–DNA complex and implications for SOS box measurement
Structure of the LexA–DNA complex and implications for SOS box measurement
Normally, expression of bacterial DNA damage repair genes is repressed by the binding of LexA protein to SOS ‘boxes’ in their operators. DNA damage activates the RecA protein, which promotes autocleavage of LexA such that its repression is relieved and repair proteins are expressed. These authors solve several structures of LexA dimer bound to SOS box DNA, and find that the orientation of the DNA-binding wings can account for the strict intersite spacing.
- Adrianna P. P. Zhang
- , Ying Z. Pigli
- & Phoebe A. Rice
-
Letter |
 Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching
Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching
The bacterial flagellar motor drives the rotation of flagellar filaments, propelling bacteria through viscous media. The rotation can switch from an anticlockwise to a clockwise direction, determining a smooth or tumbling motion. A protein called FliG forms a ring in the motor's rotor, and has been proposed to adopt distinct conformations that induce switching. Here, the full-length structure of FliG is presented, and conformational changes are identified that are involved in switching between clockwise and anticlockwise rotations.
- Lawrence K. Lee
- , Michael A. Ginsburg
- & Daniela Stock
-
Article |
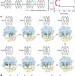 Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
During protein synthesis within the ribosome, transfer RNAs (tRNAs) move sequentially through different sites as their attached amino acids are transferred onto the growing protein chain. Large conformational movements accompany this process. Here, a staggering 1.9 million electron cryomicroscopy images of the ribosome have been processed to visualize these changes. The results reveal that the ribosome functions as a Brownian machine that couples spontaneous changes driven by thermal energy to directed movement.
- Niels Fischer
- , Andrey L. Konevega
- & Holger Stark
-
Letter |
 Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
XXXMicrotubules are nucleated in vivo by γ-tubulin complexes and comprise 13 protofilaments. How this precise geometry is controlled remains unclear. These authors report the cryo-electron microscopic structure of the universally conserved, core microtubule nucleating complex, γ-tubulin small complex. The structure provides insight into how this complex establishes thirteen-fold tubulin symmetry.
- Justin M. Kollman
- , Jessica K. Polka
- & David A. Agard
-
Letter |
 A random cell motility gradient downstream of FGF controls elongation of an amniote embryo
A random cell motility gradient downstream of FGF controls elongation of an amniote embryo
Most animal embryos grow through cell accumulation in a posterior growth zone, but the underlying forces are unknown. It is now proposed that posterior elongation in chicken embryos is an emergent property that arises from graded cell motility in random directions (as opposed to directed movement). This occurs in response to signalling through the fibroblast growth factor.
- Bertrand Bénazéraf
- , Paul Francois
- & Olivier Pourquié
-
Letter |
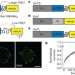 Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics
Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics
The ability of cells to respond to physical forces is central to development and physiology, but until now it has been difficult to directly measure forces across proteins in vivo. Here, however, a calibrated biosensor is described that can measure forces with high sensitivity across specific proteins in cells. This is applied to the vinculin protein, and a regulatory mechanism is revealed in which the force applied to vinculin determines whether focal adhesions assemble or disassemble.
- Carsten Grashoff
- , Brenton D. Hoffman
- & Martin A. Schwartz
-
Letter |
 Structural basis for the coupling between activation and inactivation gates in K+ channels
Structural basis for the coupling between activation and inactivation gates in K+ channels
K+ channels can convert between conductive and non-conductive forms through mechanisms that range from flicker transitions (which occur in microseconds) to C-type inactivation (which occurs on millisecond to second timescales). Here, the mechanisms are revealed through which movements of the inner gate of the K+ channel KcsA trigger conformational changes at the selectivity filter, leading to the non-conductive C-type inactivated state.
- Luis G. Cuello
- , Vishwanath Jogini
- & Eduardo Perozo
-
Letter |
 LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium
LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium
Here the authors show that in non-excitable LNCaP prostate cancer cells, the large-conductance, voltage- and calcium-activated potassium (BK) channel can be activated at negative voltages without rises in intracellular Ca2+ concentration, by interacting with an auxiliary protein, the leucine-rich repeat containing protein 26. This auxiliary protein modulates BK channel gating by enhancing the allosteric coupling between voltage-sensor activation and the channel's closed–open transition.
- Jiusheng Yan
- & Richard W. Aldrich
-
Letter |
 Subnanometre single-molecule localization, registration and distance measurements
Subnanometre single-molecule localization, registration and distance measurements
These authors have developed a method that enables them to observe single-molecule fluorescent probes with subnanometre precision and accuracy using conventional far-field fluorescence imaging. The improved resolution will enable researchers to characterize single 'molecules' of large, multisubunit biological complexes in biologically relevant environments.
- Alexandros Pertsinidis
- , Yunxiang Zhang
- & Steven Chu
-
Letter |
 Single-cell NF-κB dynamics reveal digital activation and analogue information processing
Single-cell NF-κB dynamics reveal digital activation and analogue information processing
Multicellular organisms, particularly their immune systems, rely on complex cell-to-cell communication, mediated by signalling molecules that form spatiotemporal concentration gradients. Here, high-throughput microfluidic cell culture and fluorescence microscopy, together with quantitative gene expression analysis and mathematical modelling, have been used to investigate how mammalian cells respond to different levels of TNF-α and signal to NF-κB. Both digital and analogue responses are revealed.
- Savaş Tay
- , Jacob J. Hughey
- & Markus W. Covert
-
Letter |
 Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel
Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel
Large-conductance Ca2+-gated K+ (BK) channels are essential for many biological processes, such as smooth muscle contraction and neurotransmitter release. Here, the X-ray crystal structure is presented of the entire cytoplasmic region of the human BK channel in a Ca2+-free state. Moreover, a voltage-gated K+ channel pore of known structure is 'docked' onto the gating ring to generate a structural model for the full BK channel.
- Yunkun Wu
- , Yi Yang
- & Youxing Jiang
-
Letter |
 Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability
Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability
A nerve cell sends signals to others through action potentials, which begin at the 'initial segment' of the neuron's axon. It is now shown that changes in electrical activity can alter the position of this initial segment in cultured rat hippocampal neurons. The resulting increase in intrinsic excitability — the tendency to fire action potentials — represents a new form of neuronal plasticity and could provide a new target in the control of epilepsy.
- Matthew S. Grubb
- & Juan Burrone
-
Research Highlights |
Biophysics: Molecular carnival ride
-
Letter |
 Myosin II contributes to cell-scale actin network treadmilling through network disassembly
Myosin II contributes to cell-scale actin network treadmilling through network disassembly
Eukaryotic cells crawl through a process in which the front of the cell is propelled forwards by the force provided by polymerization of actin filaments. These must be disassembled at the rear of the cell to allow sustained motility. It is now shown that non-muscle myosin II protein is needed for the disassembly of actin networks at the rear of crawling cells.
- Cyrus A. Wilson
- , Mark A. Tsuchida
- & Julie A. Theriot
-
Letter |
 A conserved spider silk domain acts as a molecular switch that controls fibre assembly
A conserved spider silk domain acts as a molecular switch that controls fibre assembly
Spider silk proteins are remarkably soluble when stored at high concentration and yet can be converted to extremely sturdy fibres, through unknown molecular mechanisms. Here, the structure of the evolutionarily conserved carboxy-terminal domain of a silk protein is presented. The results provide evidence that the structural state of this domain is essential for controlled switching between the storage and assembly forms of silk proteins. Such molecular switches might see application in the design of versatile fibrous materials.
- Franz Hagn
- , Lukas Eisoldt
- & Horst Kessler
-
News & Views |
 Transporter in the spotlight
Transporter in the spotlight
Membrane transporter proteins switch between conformational states to move substrates across membranes. The transition between these states can now be studied using single-molecule experiments.
- Nathan K. Karpowich
- & Da-Neng Wang
-
Letter |
 Designed biomaterials to mimic the mechanical properties of muscles
Designed biomaterials to mimic the mechanical properties of muscles
Here, artificial proteins are described that mimic the molecular architecture of titin — a protein that helps to govern the passive elastic properties of muscle. The new artificial proteins combine structured and unstructured domains, and can be photochemically crosslinked into a solid biomaterial that is resilient at low strains and extensible and tough at high strains. This provides an example of tailoring the macroscopic properties of a material through engineering at the single-molecule level.
- Shanshan Lv
- , Daniel M. Dudek
- & Hongbin Li
-
Letter |
 Cis-interactions between Notch and Delta generate mutually exclusive signalling states
Cis-interactions between Notch and Delta generate mutually exclusive signalling states
Notch and Delta are transmembrane proteins that allow neighbouring cells to communicate during development. Here, quantitative time-lapse microscopy has been used to show that the response of Notch to Delta on a neighbouring cell is graded, whereas its response to Delta on the same cell is sharp and occurs at a fixed threshold. A mathematical model explores how this new design principle enhances the sharpness of developmental boundaries set by classical lateral inhibition.
- David Sprinzak
- , Amit Lakhanpal
- & Michael B. Elowitz
-
Article |
 Olfactory pattern classification by discrete neuronal network states
Olfactory pattern classification by discrete neuronal network states
The brain is apt to sort sensory stimuli into discrete perceptual categories, but the neuronal activity behind this capability has been unclear. Here, the problem has been investigated by presenting zebrafish with different concentrations or types of odours. The results show that the activity of neuronal populations in the olfactory bulb is largely insensitive to changes in odour concentration, but that morphing one odour into another produces abrupt transitions between odour representations.
- Jörn Niessing
- & Rainer W. Friedrich
-
Letter |
 Dislocation nucleation governed softening and maximum strength in nano-twinned metals
Dislocation nucleation governed softening and maximum strength in nano-twinned metals
The strength of conventional metals is determined by the interaction of dislocations with obstacles such as grain boundaries. Molecular dynamics simulations reveal that the strength of ultrafine-grained copper containing twin boundaries can be controlled by a dislocation nucleation mechanism activated below a critical twin thickness. Below this thickness the material becomes softer. The smaller the grains, the smaller the critical twin boundary spacing, and the higher the metal's maximum strength.
- Xiaoyan Li
- , Yujie Wei
- & Huajian Gao
-
Letter |
 Tracking G-protein-coupled receptor activation using genetically encoded infrared probes
Tracking G-protein-coupled receptor activation using genetically encoded infrared probes
Rhodospsin is a G-protein-coupled receptor that is responsible for vision in dim light. Light isomerizes the protein's retinal chromophore and triggers concerted movements of several transmembrane helices. Here, an approach involving mutant rhodopsins and infrared spectroscopy enabled changes in the electrostatic environment to be seen as rhodopsin proceeded along its activation pathway. Early conformational changes were observed that precede the well-known larger movements of the transmembrane helices.
- Shixin Ye
- , Ekaterina Zaitseva
- & Reiner Vogel
-
News & Views |
 Joint effort bends membrane
Joint effort bends membrane
The curvature of cellular membranes is generated by proteins and lipids. A synthetic experimental system allows the interplay between protein- and lipid-generated bending mechanisms to be studied directly.
- Michael M. Kozlov
-
News & Views |
 How cilia beat
How cilia beat
Physics provides new approaches to difficult biological problems: a plausible mathematical model of how cilia and flagella beat has been formulated, but it needs to be subjected to rigorous experimental tests.
- T. J. Mitchison
- & H. M. Mitchison
-
Research Highlights |
 Molecular biology: Flowering time unravelled
Molecular biology: Flowering time unravelled

 Tiny electrostatic traps
Tiny electrostatic traps Functional roles for noise in genetic circuits
Functional roles for noise in genetic circuits