Featured
-
-
News & Views |
 More than a bystander
More than a bystander
The tendency of hydrophobic surfaces to aggregate in water is often invoked to explain how biomolecules recognize and bind to each other. Water seems to have a much more active role in these processes than had been thought.
- Philip Ball
-
Letter |
 Aspartate 112 is the selectivity filter of the human voltage-gated proton channel
Aspartate 112 is the selectivity filter of the human voltage-gated proton channel
- Boris Musset
- , Susan M. E. Smith
- & Thomas E. DeCoursey
-
Letter |
 Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase
Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase
- Yasuhito Shomura
- , Ki-Seok Yoon
- & Yoshiki Higuchi
-
Letter |
 Self-replication of information-bearing nanoscale patterns
Self-replication of information-bearing nanoscale patterns
- Tong Wang
- , Ruojie Sha
- & Nadrian C. Seeman
-
Technology Feature |
 Bright light, better labels
Bright light, better labels
The tiniest structures in cells can be seen only using sophisticated instrumentation and informatics, but what biologists really need are improved fluorescent probes.
- Monya Baker
-
Letter |
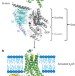 Conformational changes in the G protein Gs induced by the β2 adrenergic receptor
Conformational changes in the G protein Gs induced by the β2 adrenergic receptor
- Ka Young Chung
- , Søren G. F. Rasmussen
- & Roger K. Sunahara
-
Letter |
 Structural basis of RNA recognition and activation by innate immune receptor RIG-I
Structural basis of RNA recognition and activation by innate immune receptor RIG-I
- Fuguo Jiang
- , Anand Ramanathan
- & Joseph Marcotrigiano
-
Research Highlights |
DNA is as elastic as nylon
-
Letter |
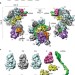 Structures of the RNA-guided surveillance complex from a bacterial immune system
Structures of the RNA-guided surveillance complex from a bacterial immune system
- Blake Wiedenheft
- , Gabriel C. Lander
- & Eva Nogales
-
Letter |
 ATP-induced helicase slippage reveals highly coordinated subunits
ATP-induced helicase slippage reveals highly coordinated subunits
- Bo Sun
- , Daniel S. Johnson
- & Michelle D. Wang
-
-
Letter |
 Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A
Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A
- A. J. Ehrlicher
- , F. Nakamura
- & T. P. Stossel
-
Article |
 Diffraction-unlimited all-optical imaging and writing with a photochromic GFP
Diffraction-unlimited all-optical imaging and writing with a photochromic GFP
- Tim Grotjohann
- , Ilaria Testa
- & Stefan W. Hell
-
Article |
 The mechanism of membrane-associated steps in tail-anchored protein insertion
The mechanism of membrane-associated steps in tail-anchored protein insertion
- Malaiyalam Mariappan
- , Agnieszka Mateja
- & Robert J. Keenan
-
Letter |
 Solution structure of a minor and transiently formed state of a T4 lysozyme mutant
Solution structure of a minor and transiently formed state of a T4 lysozyme mutant
- Guillaume Bouvignies
- , Pramodh Vallurupalli
- & Lewis E. Kay
-
Letter |
 Polar actomyosin contractility destabilizes the position of the cytokinetic furrow
Polar actomyosin contractility destabilizes the position of the cytokinetic furrow
- Jakub Sedzinski
- , Maté Biro
- & Ewa Paluch
-
Review Article |
 Dynamic molecular processes mediate cellular mechanotransduction
Dynamic molecular processes mediate cellular mechanotransduction
- Brenton D. Hoffman
- , Carsten Grashoff
- & Martin A. Schwartz
-
News & Views |
 Several small shocks beat one big one
Several small shocks beat one big one
Life-threatening abnormalities in the electrical rhythm of the heart are usually treated with the application of a large electric shock. An approach involving a significantly smaller shock energy may be equally effective. See Letter p.235
- Richard A. Gray
- & John P. Wikswo
-
Letter |
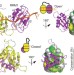 Multi-domain conformational selection underlies pre-mRNA splicing regulation by U2AF
Multi-domain conformational selection underlies pre-mRNA splicing regulation by U2AF
- Cameron D. Mackereth
- , Tobias Madl
- & Michael Sattler
-
Research Highlights |
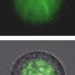 Fluorescent cells turned into lasers
Fluorescent cells turned into lasers
-
News Feature |
 Physics meets cancer: The disruptor
Physics meets cancer: The disruptor
Paul Davies likes to ask big questions. But how did the freethinking cosmologist suddenly find himself probing the physics of cancer?
- M. Mitchell Waldrop
-
Letter |
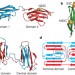 Single-molecule fluorescence reveals sequence-specific misfolding in multidomain proteins
Single-molecule fluorescence reveals sequence-specific misfolding in multidomain proteins
- Madeleine B. Borgia
- , Alessandro Borgia
- & Jane Clarke
-
Article |
 Probing cellular protein complexes using single-molecule pull-down
Probing cellular protein complexes using single-molecule pull-down
- Ankur Jain
- , Ruijie Liu
- & Taekjip Ha
-
Letter |
 Forces between clustered stereocilia minimize friction in the ear on a subnanometre scale
Forces between clustered stereocilia minimize friction in the ear on a subnanometre scale
- Andrei S. Kozlov
- , Johannes Baumgart
- & A. J. Hudspeth
-
Q&A |
 Turning point: John Dabiri
Turning point: John Dabiri
A biophysicist who linked jellyfish hydrodynamics to blood flow now turns his attention to wind energy.
- Karen Kaplan
-
Letter |
 Preserving the membrane barrier for small molecules during bacterial protein translocation
Preserving the membrane barrier for small molecules during bacterial protein translocation
- Eunyong Park
- & Tom A. Rapoport
-
Letter |
 Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue
Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue
- Yongfang Zhao
- , Daniel S. Terry
- & Jonathan A. Javitch
-
Research Highlights |
Tiny swimmers trapped by lasers
-
News |
 Dying for a long life
Dying for a long life
A chemical that stains Alzheimer's-associated proteins may help cells to cope with toxic trash.
- Ewen Callaway
-
News & Views |
 Progesterone's gateway into sperm
Progesterone's gateway into sperm
The hormone progesterone rapidly activates intracellular signalling in human sperm, regulating key aspects of their physiology. An ion channel unique to the sperm tail seems to relay progesterone's signal. See Letters p.382 & p.387 See Clarification p.598
- Steve Publicover
- & Christopher Barratt
-
Article |
 Structure and mechanism of the hexameric MecA–ClpC molecular machine
Structure and mechanism of the hexameric MecA–ClpC molecular machine
Regulated proteolysis by ATP-dependent proteases have a crucial role in protein quality control in cells. The Clp/Hsp100 proteins of the AAA+ superfamily of ATP-dependent chaperones unfold and translocate proteins into the proteolytic chamber of protease complexes. ClpC requires the adaptor protein MecA for activation and substrate targetting to the ClpCP protease complex. Here, a structural and biochemical analysis is presented of the MecA–ClpC complex revealing organizational principles and providing mechanistic insights into this complex molecular machine.
- Feng Wang
- , Ziqing Mei
- & Yigong Shi
-
News & Views |
 Flipping Watson and Crick
Flipping Watson and Crick
Watson–Crick base pairs underpin the DNA double helix. Evidence of transient changes in base-pairing geometry highlights the fact that the information held in DNA's linear sequence is stored in three dimensions. See Article p.498
- Barry Honig
- & Remo Rohs
-
News & Views |
 Push it, pull it
Push it, pull it
During migration, cells interact with their environment by exerting mechanical forces on it. A combination of two techniques shows that they do so in all three dimensions by a push–pull mechanism.
- Pascal Hersen
- & Benoît Ladoux
-
News & Views |
 A new look for the APC
A new look for the APC
Solving the structure of protein complexes is particularly challenging when they contain many subunits. In the case of the APC, a fruitful strategy has been to gain information by subtracting subunits. See Article p.227 and Letter p.274
- Ian Foe
- & David Toczyski
-
Article |
 Structural basis for the subunit assembly of the anaphase-promoting complex
Structural basis for the subunit assembly of the anaphase-promoting complex
The APC/C is a large multiprotein complex that functions as an E3 ubiquitin ligase to regulate the cell cycle. Here, the entire APC/C complex is reconstituted, and in combination with structural studies a pseudo-atomic model for 70% of the complex is provided. These results contribute towards a molecular understanding of the roles of individual subunits in APC/C assembly and their interactions with co-activators, substrates and regulatory proteins.
- Anne Schreiber
- , Florian Stengel
- & David Barford
-
Letter |
 Structural basis for site-specific ribose methylation by box C/D RNA protein complexes
Structural basis for site-specific ribose methylation by box C/D RNA protein complexes
A complex of RNA and protein known as the box C/D RNP catalyses the site-specific modification of RNAs with a 2′-O-methylation group. The structure of the full complex has now been solved, including the guide RNA and either of two substrate RNAs. This structure reveals how the guide and target RNAs are aligned, and how the methyltransferase subunit, fibrillarin, facilitates placement of the target ribose into the active site.
- Jinzhong Lin
- , Shaomei Lai
- & Keqiong Ye
-
Article |
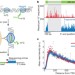 Nascent transcript sequencing visualizes transcription at nucleotide resolution
Nascent transcript sequencing visualizes transcription at nucleotide resolution
A novel technique called native elongating transcript sequencing (NET-seq) is described, which can quantify transcription with single nucleotide resolution. It is based on sequencing nascent transcripts associated with RNA polymerase II that are captured directly from live cells, and is used to gain insights into polymerase pausing and backtracking and the directionality of transcription.
- L. Stirling Churchman
- & Jonathan S. Weissman
-
Letter |
 Structure and function of an irreversible agonist-β2 adrenoceptor complex
Structure and function of an irreversible agonist-β2 adrenoceptor complex
The X-ray crystal structure of the human β2 adrenergic receptor, a G-protein-coupled receptor (GPCR), covalently bound to a small-molecule agonist is solved. Comparison of this structure with structures of this GPCR in an inactive state and in an antibody-stabilized active state reveals how binding events at both the extracellular and intracellular surfaces stabilize the active conformation of the receptor. Molecular dynamics simulations suggest that the agonist-bound active state spontaneously relaxes to an inactive-like state in the absence of a G protein.
- Daniel M. Rosenbaum
- , Cheng Zhang
- & Brian K. Kobilka
-
Letter |
 Sensing the anomeric effect in a solvent-free environment
Sensing the anomeric effect in a solvent-free environment
The anomeric effect is a chemical phenomenon that refers to an observed stabilization of six-membered carbohydrate rings when they contain an electronegative substituent at the C1 position of the ring. This stereoelectronic effect influences the three-dimensional shapes of many biological molecules, but the underlying physical origin is unclear. Here it is shown that complexes formed between a truncated peptide motif and an isolated sugar in the gas phase are nearly identical structurally; however, the strength of the polarization of their interactions with the peptide differs greatly. It will be important to re-evaluate the influence, and biological effects, of substituents at position C2 of the six-membered carbohydrate rings.
- Emilio J. Cocinero
- , Pierre Carcabal
- & Benjamin G. Davis
-
Letter |
 Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
The initial crystal structure of LeuT, together with subsequent functional and structural studies, provided direct evidence for a single, high-affinity substrate-binding site. Recent binding, flux and molecular simulation studies, however, have been interpreted in terms of a model where there are two high-affinity binding sites: the second (S2) site is believed to be located within the extracellular vestibule. Here, direct measurement is performed of substrate binding to wild-type LeuT and to S2 site mutants using isothermal titration calorimetry, equilibrium dialysis and scintillation proximity assays. The conclusion is made that LeuT harbours a single, centrally located, high-affinity substrate-binding site.
- Chayne L. Piscitelli
- , Harini Krishnamurthy
- & Eric Gouaux
-
Letter |
 Distributed biological computation with multicellular engineered networks
Distributed biological computation with multicellular engineered networks
For synthetic biologists' creativity to be unleashed, basic circuits must become truly interchangeable, that is, modular and scalable. This study, one of two linked papers, has harnessed yeast pheromone communication to achieve complex computation through communication between individual cells performing simple logic functions. Such extracellular 'chemical wiring' is one promising way to get around intracellular noise when building more complex genetic circuitry.
- Sergi Regot
- , Javier Macia
- & Ricard Solé
-
Letter |
 The mechanism of sodium and substrate release from the binding pocket of vSGLT
The mechanism of sodium and substrate release from the binding pocket of vSGLT
Here, a comprehensive study of the sodium/galactose transporter (vSGLT) is presented, consisting of molecular dynamics simulations, biochemical characterization and a new crystal structure of the 'inward-open' conformation. These experiments show that sodium exit causes a reorientation of transmembrane helix 1, opening an inner gate required for substrate exit, while also triggering minor rigid-body movements in two sets of transmembrane helical bundles. This cascade of conformational changes is responsible for the proper timing of ion and substrate release.
- Akira Watanabe
- , Seungho Choe
- & Jeff Abramson
-
Letter |
 Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
During translation, tRNAs enter the ribosome and then move sequentially through three sites, known as A, P and E, as they transfer their attached amino acids onto the growing peptide chain. How the ribosome facilitates tRNA translocation between the sites remains largely unknown. Now a study uses multiparticle cryoelectron microscopy of a ribosome bound to the translation elongation factor, EF-G, to get information about tRNA movement. It identifies two new substates and sees that translocation is linked to unratcheting of the 30S ribosomal subunit.
- Andreas H. Ratje
- , Justus Loerke
- & Christian M. T. Spahn
-
Letter |
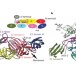 Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography
Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography
The E1 and E2 glycoproteins of alphaviruses form heterodimers and assemble into spikes on the virus surface, which mediate receptor binding and endocytosis. When the virion encounters acidic pH in the endosome E1 and E2 dissociate and E1 triggers fusion with the endosomal membrane. Two papers now provide the first crystal structures for glycoprotein complexes incorporating E2 at acidic and neutral pH, respectively. Together they provide insight into how fusion activation is controlled in alphaviruses.
- James E. Voss
- , Marie-Christine Vaney
- & Félix A. Rey
-
Letter |
 Single-molecule imaging reveals mechanisms of protein disruption by a DNA translocase
Single-molecule imaging reveals mechanisms of protein disruption by a DNA translocase
Protein machineries that move along the DNA, such as DNA polymerases and helicases, will necessarily encounter other bound proteins interacting with specific sites. Using 'curtains' of labelled DNA, this study measured whether such bound proteins interfere with the activity of the bacterial DNA translocase RecBCD. The translocase is able to push the proteins over nonspecific sites for thousands of base pairs before they are displaced.
- Ilya J. Finkelstein
- , Mari-Liis Visnapuu
- & Eric C. Greene
-
Letter |
 Nanoscale architecture of integrin-based cell adhesions
Nanoscale architecture of integrin-based cell adhesions
Focal adhesions link the extracellular matrix by integrin receptors to cytoplasmic actin filaments and are fundamental to human physiology. These authors determine the molecular architecture of focal adhesions by mapping protein organization at the nanoscale level. The results demonstrate that focal adhesions possess a well-organized ultrastructure made up of at least three spatial and functional compartments that mediate their interdependent functions.
- Pakorn Kanchanawong
- , Gleb Shtengel
- & Clare M. Waterman
-
Letter |
 Tension directly stabilizes reconstituted kinetochore-microtubule attachments
Tension directly stabilizes reconstituted kinetochore-microtubule attachments
The kinetochore is a large protein complex that assembles on centromeric DNA and captures microtubules to mediate chromosome separation. These authors report the first purification of functional kinetochores. They also show that kinetochore particles maintain load-bearing associations with assembling and disassembling ends of single microtubules and that tension increases the lifetimes of the attachments directly. These results provide evidence that tension selectively stabilises kinetochore–microtubule interactions.
- Bungo Akiyoshi
- , Krishna K. Sarangapani
- & Sue Biggins
-
Letter |
 Interdependence of behavioural variability and response to small stimuli in bacteria
Interdependence of behavioural variability and response to small stimuli in bacteria
In his study of Brownian motion, Einstein realized that the same random molecular movements characterizing a substance at rest should affect, for example, the drag it opposes to a particle pushed through it. This was later generalized as the fluctuation–response theorem (FRT), but whether and how it may apply to biological systems, which operate far from equilibrium, has remained an open question. Based on the unmatched fine-scale measurements possible in the study of bacterial chemotaxis, it is now revealed that the FRT does apply in this case, and ways to dissect which features in the biochemical network couple its internal states with its responses to external stimuli are suggested.
- Heungwon Park
- , William Pontius
- & Philippe Cluzel
-
Letter |
 The proteasome antechamber maintains substrates in an unfolded state
The proteasome antechamber maintains substrates in an unfolded state
The proteasome is a multi-protein complex that enzymatically degrades proteins. Proteolysis occurs in a barrel-shaped 20S core particle comprising three interconnected cavities, including a pair of antechambers in which substrates are held before degradation. These authors demonstrate that substrates interact actively with the antechamber walls and that the environment in this compartment is optimized to maintain the substrates in unfolded states so as to be accessible for hydrolysis.
- Amy M. Ruschak
- , Tomasz L. Religa
- & Lewis E. Kay

 Turning point: Josefina del Mármol
Turning point: Josefina del Mármol Crystal structure of nucleotide-free dynamin
Crystal structure of nucleotide-free dynamin