« Prev Next »
Aging is an Evolutionary Paradox
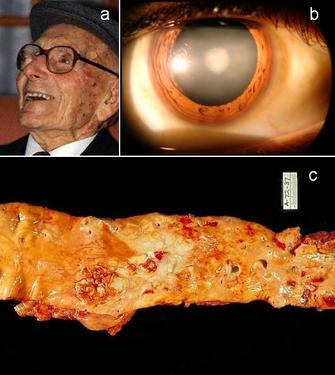
Why do we age and die? Aging, or senescence as it is sometimes called, is an inevitable progressive deterioration of physiological function with increasing age, demographically characterized by an age-dependent increase in mortality and decline of fecundity (Rose 1991, Bronikowksi & Flatt 2010, see Figure 1). This poses an evolutionary paradox: natural selection designs organisms for optimal survival and reproductive success (Darwinian fitness), so why does evolution not prevent aging in the first place?
For centuries, beginning with Aristotle, scientists and philosophers have struggled to resolve this enigma. The Roman poet and philosopher Lucretius, for example, argued in his De Rerum Natura (On the Nature of Things) that aging and death are beneficial because they make room for the next generation (Bailey 1947), a view that persisted among biologists well into the 20th century. The famous 19th century German biologist, August Weissmann, for instance, suggested – similar to Lucretius – that selection might favor the evolution of a death mechanism that ensures species survival by making space for more youthful, reproductively prolific individuals (Weissmann 1891). But this explanation turns out to be wrong. Since the cost of death to individuals likely exceeds the benefit to the group or species, and because long-lived individuals leave more offspring than short-lived individuals (given equivalent reproductive output), selection would not favor such a death mechanism.
A more parsimonious evolutionary explanation for the existence of aging therefore requires an explanation that is based on individual fitness and selection, not on group selection. This was understood in the 1940's and 1950's by three evolutionary biologists, J.B.S. Haldane, Peter B. Medawar and George C. Williams, who realized that aging does not evolve for the "good of the species". Instead, they argued, aging evolves because natural selection becomes inefficient at maintaining function (and fitness) at old age. Their ideas were later mathematically formalized by William D. Hamilton and Brian Charlesworth in the 1960's and 1970's, and today they are empirically well supported. Below we review these major evolutionary insights and the empirical evidence for why we grow old and die.
For further in-depth coverage of the evolution of aging we point the reader to Rose (1991), Hughes & Reynolds (2005), Promislow & Bronikowski (2006), Flatt & Schmidt (2009), and references therein. Also see Rauschert (2010) and Shefferson (2010) in Nature Education Knowledge.
The Force of Selection Declines with Age
As mentioned above, the key conceptual insight that allowed Medawar, Williams, and others, to develop the evolutionary theory of aging is based on the notion that the force of natural selection, a measure of how effectively selection acts on survival rate or fecundity as a function of age, declines with progressive age (see Hamilton 1966, Charlesworth 2000, Rose et al. 2007) (Figure 2). This was first noted, though not formally analyzed, by Fisher in his famous book The Genetical Theory of Natural Selection (1930), and both Haldane (1941) and Medawar (1946, 1952) came to the same conclusion. Haldane (1941) proposed that the declining strength of selection with age might explain the relatively high prevalence of the dominant allele causing Huntington’s disease: he speculated that, since Huntington's typically only affects people beyond age 30, such a disease would not have been efficiently eliminated by selection in ancestral, pre-modern populations because most people would already have died well before they could experience this late-onset disease. Thus, the disease would not have been "seen" by, or subject to, selection.
Based on Fisher's and Haldane's ideas, Medawar (1946, 1952) worked out the first complete verbal and graphical model of how aging evolves (also see next section). The gist of Medawar's argument is as follows. First, for most organisms, the natural world is dangerous since it abounds with competitors, predators, pathogens, accidents, and other hazards. It follows from this that in natural populations most individuals die or get killed before they can grow old and suffer the symptoms of aging: thus, individuals have a very small overall probability of being alive and reproductive at an advanced age (e.g., Moorad & Promislow 2010). Second, the strength of natural selection declines with increasing age (Figure 2), such that selection ignores the performance of individuals late in life. As a consequence, selection is unable to favor beneficial effects, or to counteract deleterious effects, when these effects are expressed at advanced ages. For example, if a beneficial or deleterious mutation occurs only after reproduction has ceased, then it will not affect fitness (reproductive success) and can therefore not be efficiently selected for or against. However, even if a mutation occurs earlier, say during the reproductive period, its effects may not be visible to selection since, if extrinsic, environmentally imposed mortality is high, individuals that could express the mutation are likely to be dead already.
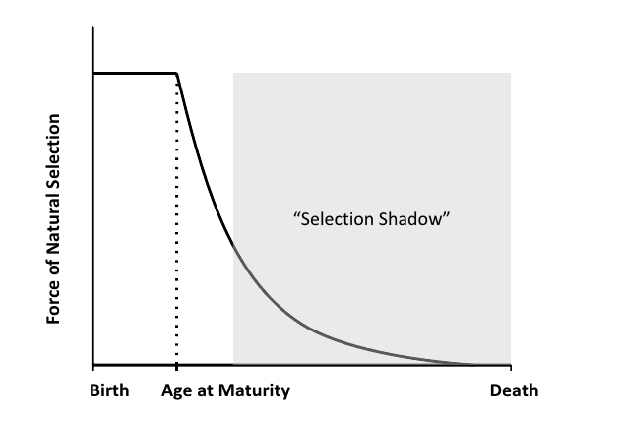
Medawar (1946, 1952) and Williams (1957) realized that these deductions, later mathematically expressed by Hamilton (1966, also see Rose et al. 2007), would open the door for the evolution of aging.
The Mutation Accumulation Hypothesis
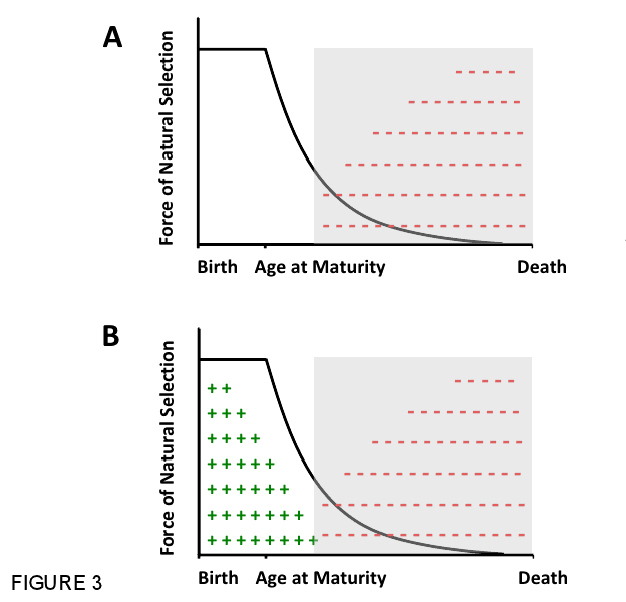
Following the logic outlined above, Medawar (1946, 1952) reasoned that, if the effects of a deleterious mutation were restricted to late ages, when reproduction has largely stopped and future survival is unlikely, carriers of the negative mutation would have already passed it on to the next generation before the negative late-life effects would become apparent. In such a situation, natural selection would be weak and inefficient at eliminating such a mutation, and over evolutionary time such effectively neutral mutations would accumulate in the population by genetic drift, which in turn would lead to the evolution of aging. This is known as Medawar's mutation accumulation (MA) hypothesis (Figure 3A). The effects of such a mutation accumulation process would only become manifest at the organismal level after the environment changes such that individuals experience less extrinsic mortality (e.g., due to decreased predation) and thus live to an age where they actually express the symptoms of aging.
Medawars' MA hypothesis was later put on firm mathematical ground by Charlesworth (1994, 2001). Several experimental studies, mainly in fruit flies (Drosophila melanogaster), have found – somewhat limited – empirical support for the occurrence of MA (see Hughes & Reynolds 2005, Charlesworth 1994, Hughes et al. 2002 for a discussion).
The Antagonistic Pleiotropy Hypothesis
In an influential paper published in Evolution, George C. Williams (1957) took Medawar's ideas a step further. If it is true that selection cannot counteract deleterious effects at old age, he argued, then mutations or alleles might exist that have opposite, pleiotropic effects at different ages: genetic variants that on the one hand exhibit beneficial effects on fitness early in life, when selection is strong, but that on the other hand have deleterious effects late in life, when selection is already weak. This idea is known today as the antagonistic pleiotropy (AP) hypothesis for the evolution of aging (see Rose 1991, Flatt & Promislow 2007, Figure 3B). Williams pointed out that, if the beneficial effects of such mutations early in life outweigh their deleterious effects at advanced age, such genetic variants would be favored and enriched in a population, thus leading to the evolution of aging. Thus, under Williams' hypothesis, the evolution of aging can be seen as a maladaptive byproduct of selection for survival and reproduction during youth.
A fundamental corollary of Williams’ AP hypothesis is that early fitness components such as reproduction should genetically trade-off with late fitness components such as survival at old age, so that, for example, genotypes with high early fecundity should be shorter lived than those with low reproduction (e.g., Williams 1957, Rose 1991, Charlesworth 1994, Hughes & Reynolds 2005). In a somewhat similar vein, Kirkwood’s 1977 "disposable soma" (DS) hypothesis predicts that the optimal level of investment into somatic maintenance and repair will evolve to be below that required for indefinite survival. The idea here is that the evolution of a higher investment is unlikely to pay off since the return from such an investment may never be realized due to extrinsic mortality. Moreover, investment into reproduction – or early fitness components in general – might withdraw limited resources that could otherwise be used for somatic maintenance and repair. Such resource allocation trade-offs can thus been seen as a physiological extension of Williams' AP model.
Although the relative frequency of MA versus AP is still debated (both may typically go hand in hand - see also Moorad & Promislow 2009), there is robust evidence today for the existence of fitness trade-offs that are consistent with the notion of AP (for a recent discussion of the positive evidence see Flatt & Promislow 2007, and Flatt 2011, but also see Moorad & Promislow 2009). Whether such trade-offs are physiologically caused by competitive energy or resource allocation – as would be expected under the DS hypothesis – remains somewhat controversial, but the trade-offs themselves are well established (see Flatt 2011). Most importantly, the kinds of trade-offs postulated by Williams, have been found at the evolutionary level: for example, fruit flies that were artificially selected for increased late-life reproductive success were found to be long-lived at the expense of reduced early fecundity in several, now classical, experiments in the labs of Michael Rose and Leo Luckinbill (Rose & Charlesworth 1980, Rose 1984, Luckinbill et al. 1984). These elegant experiments represent the first solid empirical tests of the evolutionary theory of aging (Rose 1991).
The classical evolutionary theory of aging has therefore two fundamental cornerstones: MA and AP. However, it is worth noting that both models are conceptually very similar: under MA, aging evolves through the accumulation of effectively neutral mutations with deleterious late-life effects, whereas, under AP, aging occurs due to mutations with beneficial early- and deleterious late-life effects. In reality, probably both types of mutations occur in populations, yet their relative frequencies remain unknown. Furthermore, the age distribution of mutational effects may be much more complicated than these two scenarios suggest (e.g., Moorad & Promislow 2008).
Evolution of Lifespan

Different organisms vary dramatically in their lifespan (Figure 4). Obviously, aging negatively affects the duration of life since it increases the risk of death. These intrinsic, maladaptive effects of aging, unchecked by selection, are, however, not the only factors affecting the length of life. Independent of whether aging occurs or not, reproductive lifespan can evolve adaptively in response to selection for increased reproductive success (Stearns 1992). A longer lifespan normally implies increased reproductive success, and factors such as low adult mortality (permitting more reproductive events per lifetime), high juvenile mortality (making it necessary for adults to reproductively compensate for such loss), and high variation in juvenile mortality from one bout of reproduction to the next (increasing uncertainty in reproductive success and requiring reproductive compensation as well) therefore all tend to lengthen reproductive lifespan (Stearns 1992). These lifespan promoting effects of selection are balanced by those that tend to increase adult mortality relative to juvenile mortality. Consequently, if extrinsic, environmentally imposed adult mortality is high, selection becomes weak, thereby allowing the evolution of higher levels of intrinsic mortality (i.e., aging). Moreover, even though selection might favor increased reproductive success, and thus a longer reproductive lifespan, the length of life might be limited by intrinsic trade-offs between reproduction and survival caused by AP. Thus, the evolution of lifespan can be viewed as a balance between selection for increased reproductive success and the factors that increase the intrinsic age-dependent components of mortality (Stearns 1992).
These ideas have been empirically tested and corroborated by several researchers. For example, using an elegant experimental evolution design, Stearns et al. (2000) exposed fruit flies to either high or low levels of extrinsic adult mortality (HAM versus LAM) and found that LAM flies evolved significantly lower levels of intrinsic mortality relative to HAM flies: in other words, HAM flies evolved more rapid aging than LAM flies.
Given that there is ample genetic variation for lifespan and the rate of aging, and given that aging can readily evolve by MA and/or AP, is aging then likely to be universal among species? Clearly, there is a remarkable amount of variation in lifespan among different species, including some extremely short-lived as well long-lived species (e.g., Finch 1990, Figure 4). A lot of this diversity in lifespan can be quite readily explained by variation in the levels of extrinsic mortality and the evolution of different optimal lengths of reproductive life, including the existence of semelparous organisms that reproduce only once and then die (Stearns 1992). For example, species that are well protected from predators – for example, those that have a shell, can fly, or are poisonous – tend to live longer than related, less well-protected species (e.g., Austad & Fischer 1991, Blanco & Sherman 2005). But are there immortal organisms? Although examples of organisms that age very slowly are well known (e.g., Finch 1990, see Figure 4), it is not yet sufficiently clear whether there exist species that truly do not age at all. Bacteria are a good case in point.
For a long time it was thought that bacteria do not age. Indeed, one of Williams' (1957) strongest assertions about the evolution of aging was that only organisms with a separation of germ line and soma should age. In such organisms, the germ line is maintained indefinitely, but the aging soma is “disposable” after fulfilling its reproductive role. Bacteria, by contrast, do not exhibit a clear delineation into germ line and soma, and should therefore be immortal. More important than this lack of a clear germ line/soma distinction, however, is the fact that prokaryotes, protozoans, algae, and symmetrically dividing unicells, do not have clearly delineated age classes (Rose 1991, Partridge & Barton 1993). In symmetrically dividing unicells, for example, individuals should not age because parent and offspring are phenotypically indistinguishable – it is impossible to determine old from young, and age is thus invisible to selection. By the same logic, aging should exist in asymmetrically reproducing organisms where aging parents are phenotypically distinct from offspring.
Indeed, an asymmetrically dividing bacterium has recently been found to show senescence (Ackermann et al. 2003). Remarkably, however, even the symmetrically dividing E. coli ages: it shows subcellular mother-offspring asymmetry, delineating age classes upon which selection can act to produce senescence (Stewart et al. 2005). Moreover, Ackermann et al. (2007) modeled the origin of aging in the history of life and found that, even when cells divide symmetrically, unicellulars readily evolve a state of asymmetric, unequal distribution of cellular damage among daughter cells. However, as soon as such an asymmetry evolves, aging evolves. Thus, aging – despite remarkable variation in the duration of life among different species – might be a fundamental and inevitable property of cellular life.
Summary
We have introduced what evolutionary biologists think about the evolution of aging. Today, it is clear that aging is not a positively selected, programmed death process, and has not evolved for "the good of the species". Instead, aging is a feature of life that exists because selection is weak and ineffective at maintaining survival, reproduction, and somatic repair at old age. Based on the observation that the force of selection declines as a function of age, two main hypotheses have been formulated to explain why organisms grow old and die: the mutation accumulation (MA) and the antagonistic pleiotropy (AP) hypotheses. Under MA, aging evolves because selection cannot efficiently eliminate deleterious mutations that manifest themselves only late in life. Under AP, aging evolves as a maladaptive byproduct of selection for increased fitness early in life, with the beneficial early-life effects being genetically coupled to deleterious late-life effects that cause aging. Aging clearly shortens lifespan, but lifespan is also shaped by selection for an increased number of lifetime reproductive events. The evolution of lifespan is therefore a balance between selective factors that extend the reproductive period and components of intrinsic mortality that shorten it. Whether there exist truly immortal organisms is controversial, and recent evidence suggests in fact that aging might be an inevitable property of all cellular life.
Glossary
Fecundity - Fecundity is defined as the number of offspring (e.g., gametes, eggs, propagules) or the rate of offspring production (e.g., the number of eggs laid per female per unit time).
Fitness - Fitness (sometimes also called Darwinian fitness) is a measure of the relative expected contribution of a genotype (or phenotype) to future generations. The easiest way to think about fitness is in terms of lifetime reproductive success of a genotype (or phenotype) relative to other such types in a population. Note that natural selection can be defined as heritable variation among genotypes in fitness.
Germ line - The germ line is a specialized lineage of stem cells that gives rise to gametes (eggs, sperm).
Parsimony, parsimonious - The principle of parsimony (sometimes also called Occam's razor) states that when choosing among several competing explanations (or models, or hypotheses) to explain a particular phenomenon it is often best to select the simplest (i.e., making the fewest assumptions). If new evidence becomes available the explanation can be re-evaluated against the facts: if the simplest explanation still explains the facts best, it should be retained. However, if the new evidence suggests that a more complex explanation has better explanatory power, then the simpler alternative should be discarded.
Pleiotropy, pleiotropic - Pleiotropy means that a gene (or allele or mutation) affects two or more traits (or processes or functions).
Semelparity, semelparous - Semelparous organisms are those that only have one reproductive event per lifetime (independent of how many offspring are produced in this single event). Semelparity is sometimes also called "big bang" reproduction.
Senescence - Senescence is essentially synonymous with aging, i.e. the age-dependent decline in physiological function, ultimately leading to death. At the demographic level, this physiological deterioration is manifest as a decline in fecundity and an increase in mortality with increasing age.
Soma - The non-reproductive parts of the body (and its organs, tissues, and cells) that carry out all biological functions except reproduction. The soma is typically contrasted with the germ line, i.e. the lineage of cells that gives rise to gametes, and the reproductive organs.
References and Recommended Reading
Ackermann, M. et al. On the evolutionary origin of aging. Aging Cell 6, 235–244 (2007).
Austad, S. N. & Fischer, K. E. Mammalian aging, metabolism, and ecology: Evidence from the bats and marsupials. Journal of Gerontology 46, B47–B53 (1991).
Bailey, C. Titi Lucreti Cari De Rerum Natura. Volume 3, Oxford, UK: Clarendon Press, 1947.
Blanco, M. A. & Sherman, P. W. Maximum longevities of chemically protected and non-protected fishes, reptiles, and amphibians support evolutionary hypotheses of aging. Mechanisms of Ageing and Development 126, 794–803 (2005).
Bronikowski, A. M. & Flatt, T. Aging and its demographic measurement. Nature Education Knowledge 1, 3 (2011).
Charlesworth, B. Evolution in Age-Structured Populations. Cambridge, UK: Cambridge University Press, 1994.
Charlesworth, B. Fisher, Medawar, Hamilton and the evolution of aging. Genetics 156, 927–931 (2000).
Charlesworth, B. Patterns of age-specific means and genetic variances of mortality rates predicted by the mutation accumulation theory of aging. Journal of Theoretical Biology 210, 47–65 (2001).
Finch, C. E. Longevity, Senescence and the Genome. Chicago, IL: University of Chicago Press, 1990.
Fisher, R. A. The Genetical Theory of Natural Selection. Oxford, UK: Clarendon Press, 1930.
Flatt, T. Survival costs of reproduction in Drosophila. Experimental Gerontology, In Press (2011).
Flatt, T. & Promislow, D. E. L. Physiology: Still pondering an age-old question. Science 318, 1255–1256 (2007).
Flatt, T. & Schmidt, P. S. Integrating evolutionary and molecular genetics of aging. Biochimica et Biophysica Acta 1790, 951–962 (2009).
Haldane, J. B. S. New Paths in Genetics. London, UK: Allen & Unwin, 1941.
Hamilton, W. D. The moulding of senescence by natural selection. Journal of Theoretical Biology 12, 12–45 (1966).
Hughes, K. A. & Reynolds, R. M. Evolutionary and mechanistic theories of aging. Annual Review of Entomology 50, 421–445 (2005).
Hughes, K. A. et al. A test of evolutionary theories of aging. Proceedings of the National Academy of Sciences of the United States of America 99, 14286–14291 (2002).
Kirkwood, T. B. L. Evolution of ageing. Nature 270, 301–304 (1977).
Luckinbill, L. S. et al. Selection for delayed senescence in Drosophila melanogaster. Evolution 38, 996–1003 (1984).
Medawar, P. B. Old age and natural death. Modern Quarterly 1, 30–56 (1946).
Medawar, P. B. An Unsolved Problem of Biology. London, UK: H. K. Lewis, 1952.
Moorad, J. A. & Promislow, D. E. L. What can genetic variation tell us about the evolution of senescence? Proceedings of the Royal Society B: Biological Sciences 276, 2271–2278 (2009).
Moorad, J. A. & Promislow, D. E. L. Evolution: Aging up a tree? Current Biology 20, R406–R408 (2010).
Partridge, L. & Barton, N. H. Optimality, mutation and the evolution of ageing. Nature 362, 305–311 (1993).
Promislow, D. E. L. & Bronikowski, A. "Evolutionary genetics of senescence," in Evolutionary Genetics: Concepts and Case Studies, eds. C. W. Fox & J. B. Wolf (Oxford University Press, 2006) 464–481.
Rauschert, E. Survivorship curves. Nature Education Knowledge 1, 18 (2010).
Rose, M. R. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38, 1004–1010 (1984).
Rose, M. R. Evolutionary Biology of Aging. New York, NY: Oxford University Press, 1991.
Rose, M. R. & Charlesworth, B. A test of evolutionary theories of senescence. Nature 287, 141–142 (1980).
Rose, M. R. et al. Hamilton's forces of natural selection after forty years. Evolution 61, 1265–1276 (2007).
Shefferson, R. P. Why are life histories so variable? Nature Education Knowledge 1, 1 (2010).
Stearns, S. C. The Evolution of Life Histories. Oxford, UK: Oxford University Press, 1992.
Stearns, S. C. et al. Experimental evolution of aging, growth, and reproduction in fruitflies. Proceedings of the National Academy of Sciences of the United States of America 97, 3309–3313 (2000).
Stewart, E. J. et al. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biology 3, 295–300 (2005).
Weissmann, A. Essays on Heredity. Oxford, UK: Clarendon Press, 1891.
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).