Abstract
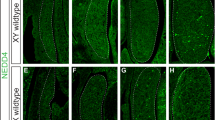
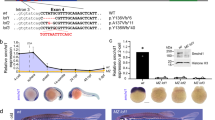
In most mammals, male development is triggered by the transient expression of the Y-chromosome gene, Sry, which initiates a cascade of gene interactions ultimately leading to the formation of a testis from the indifferent fetal gonad1,2,3,4. Several genes5,6,7,8, in particular Sox9, have a crucial role in this pathway9,10,11,12,13,14. Despite this, the direct downstream targets of Sry and the nature of the pathway itself remain to be clearly established15,16. We report here a new dominant insertional mutation, Odsex (Ods), in which XX mice carrying a 150-kb deletion (approximately 1 Mb upstream of Sox9) develop as sterile XX males lacking Sry. During embryogenesis, wild-type XX fetal gonads downregulate Sox9 expression, whereas XY and XX Ods/+ fetal gonads upregulate and maintain its expression13,14. We propose that Ods has removed a long-range, gonad-specific regulatory element that mediates the repression of Sox9 expression in XX fetal gonads. This repression would normally be antagonized by Sry protein in XY embryos. Our data are consistent with Sox9 being a direct downstream target of Sry and provide genetic evidence to support a general repressor model of sex determination in mammals17,18.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sinclair, A.H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990).
Gubbay, J. et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346, 245–250 (1990).
Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121 (1991).
Berta, P. et al. Genetic evidence equating SRY and the testis-determining factor. Nature 348, 448–450 (1990).
Foster, J.W. et al. Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials. Nature 359, 531–533 (1992).
Moniot, B., Berta, P., Scherer, G., Sudbeck, P. & Poulat, F. Male specific expression suggests role of DMRT1 in human sex determination. Mech. Dev. 91, 323–325 (2000).
De Grandi, A. et al. The expression pattern of a mouse doublesex-related gene is consistent with a role in gonadal differentiation. Mech. Dev. 90, 323–326 (2000).
Raymond, C.S. et al. Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691–695 (1998).
Foster, J.W. et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530 (1994).
Wagner, T. et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120 (1994).
Mansour, S., Hall, C.M., Pembrey, M.E. & Young, I.D. A clinical and genetic study of campomelic dysplasia. J. Med. Genet. 32, 415–420 (1995).
Huang, B., Wang, S., Ning, Y., Lamb, A. & Bartley, J. Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 87, 349–353 (1999).
Kent, J., Wheatley, S.C., Andrews, J.E., Sinclair, A.H. & Koopman, P. A male-specific role for SOX9 in vertebrate sex determination. Development 122, 2813–2822 (1996).
Morais da Silva, S. et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nature Genet. 14, 62–68 (1996).
Swain, A. & Lovell-Badge, R. Mammalian sex determination: a molecular drama. Genes Dev. 13, 755–767 (1999).
Capel, B. Sex in the 90s: SRY and the switch to the male pathway. Annu. Rev. Physiol. 60, 497–523 (1998).
Graves, J.A. Interactions between SRY and SOX genes in mammalian sex determination. Bioessays 20, 264–269 (1998).
McElreavey, K., Vilain, E., Abbas, N., Herskowitz, I. & Fellous, M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc. Natl Acad. Sci. USA 90, 3368–3372 (1993).
Yokoyama, T. et al. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 18, 7293–7298 (1990).
Bishop, C.E. & Mitchell, M.J. Encyclopedia of the mouse genome VII. Mouse chromosome Y. Mamm. Genome 8, S378–381 (1998).
Burgoyne, P.S. The mammalian Y chromosome: a new perspective. Bioessays 20, 363–366 (1998).
Sutcliffe, M.J. & Burgoyne, P.S. Analysis of the testes of H-Y negative XOSxrb mice suggests that the spermatogenesis gene (Spy) acts during the differentiation of the A spermatogonia. Development 107, 373–380 (1989).
Bi, W., Deng, J.M., Zhang, Z., Behringer, R.R. & de Crombrugghe, B. Sox9 is required for cartilage formation. Nature Genet. 22, 85–89 (1999).
Pfeifer, D. et al. Campomelic dysplasia translocation breakpoints are scattered over 1 Mb proximal to SOX9: evidence for an extended control region. Am. J. Hum. Genet. 65, 111–124 (1999).
Capel, B. et al. Deletion of Y chromosome sequences located outside the testis determining region can cause XY female sex reversal. Nature Genet. 5, 301–307 (1993).
Zhao, S. & Overbeek, P.A. Tyrosinase-related protein 2 promoter targets transgene expression to ocular and neural crest-derived tissues. Dev. Biol. 216, 154–163 (1999).
Rowe, L.B. et al. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource. Mamm. Genome 5, 253–274 (1994).
Hustert, E., Scherer, G., Olowson, M., Guenet, J.L. & Balling, R. Rbt (Rabo torcido), a new mouse skeletal mutation involved in anteroposterior patterning of the axial skeleton, maps close to the Ts (tail-short) locus and distal to the Sox9 locus on chromosome 11. Mamm. Genome 7, 881–885 (1996).
Zhao, Q., Eberspaecher, H., Lefebvre, V. & De Crombrugghe, B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. 209, 377–386 (1997).
Acknowledgements
We thank G. Schuster for microinjections; L. Vien for animal husbandry and PCR assays; B. de Crombrugghe for the Sox9 in situ hybridization probe; H. Boettger-Tong for advice on several aspects of the work; and B. Capel and P. Koopman for discussion on the manuscript. Supported by grants from the National Institutes of Health (to C.E.B., R.R.B. and P.A.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bishop, C., Whitworth, D., Qin, Y. et al. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet 26, 490–494 (2000). https://doi.org/10.1038/82652
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/82652
This article is cited by
-
Mapping of a responsible region for sex reversal upstream of Sox9 by production of mice with serial deletion in a genomic locus
Scientific Reports (2018)
-
Vertebrate sex determination: evolutionary plasticity of a fundamental switch
Nature Reviews Genetics (2017)
-
Copy number variation in the region harboring SOX9 gene in dogs with testicular/ovotesticular disorder of sex development (78,XX; SRY-negative)
Scientific Reports (2015)