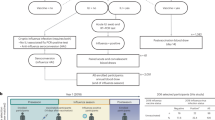
Abstract
Influenza A viruses remain a global threat to human health, with continued pandemic potential. In this Review, we discuss our current understanding of the optimal immune responses that drive recovery from influenza virus infection, highlighting the fine balance between protective immune mechanisms and detrimental immunopathology. We describe the contribution of innate and adaptive immune cells, inflammatory modulators and antibodies to influenza virus-specific immunity, inflammation and immunopathology. We highlight recent human influenza virus challenge studies that advance our understanding of susceptibility to influenza and determinants of symptomatic disease. We also describe studies of influenza virus-specific immunity in high-risk groups following infection and vaccination that inform the design of future vaccines to promote optimal antiviral immunity, particularly in vulnerable populations. Finally, we draw on lessons from the COVID-19 pandemic to refocus our attention to the ever-changing, highly mutable influenza A virus, predicted to cause future global pandemics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Iuliano, A. D. et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300 (2018).
GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 7, 69–89 (2019).
Kang, M., Zanin, M. & Wong, S. S. Subtype H3N2 influenza A viruses: an unmet challenge in the western Pacific. Vaccines 10, 112 (2022).
Pendrey, C. G. et al. The re-emergence of influenza following the COVID-19 pandemic in Victoria, Australia, 2021 to 2022. Eur. Surveill. 28, 2300118 (2023).
Zhu, W. & Gu, L. Clinical, epidemiological, and genomic characteristics of a seasonal influenza A virus outbreak in Beijing: a descriptive study. J. Med. Virol. 95, e29106 (2023).
Dhanasekaran, V. et al. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat. Commun. 13, 1721 (2022).
Australian influenza surveillance report. Australian Government Department of Health and Aged Care https://www.health.gov.au/sites/default/files/2023-10/aisr-fortnightly-report-no-14-2-october-to-15-october-2023.pdf (2023).
Caini, S. et al. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS ONE 14, e0222381 (2019).
Ambrose, C. S. & Levin, M. J. The rationale for quadrivalent influenza vaccines. Hum. Vaccin. Immunother. 8, 81–88 (2012).
Short, K. R., Kedzierska, K. & van de Sandt, C. E. Back to the future: lessons learned from the 1918 influenza pandemic. Front. Cell Infect. Microbiol. 8, 343 (2018).
Krammer, F. et al. Influenza. Nat. Rev. Dis. Prim. 4, 3 (2018).
Gao, R. et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 1888–1897 (2013).
Wille, M. & Klaassen, M. No evidence for HPAI H5N1 2.3.4.4b incursion into Australia in 2022. Influenza Other Respir. Viruses 17, e13118 (2023).
Gilbertson, B. & Subbarao, K. Mammalian infections with highly pathogenic avian influenza viruses renew concerns of pandemic potential. J. Exp. Med. 220, e20230447 (2023).
Kalil, A. C. & Thomas, P. G. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit. Care 23, 258 (2019).
Tran, D. et al. Hospitalization for influenza A versus B. Pediatrics 138, e20154643 (2016).
Puig-Barbera, J. et al. Influenza epidemiology and influenza vaccine effectiveness during the 2015-2016 season: results from the Global Influenza Hospital Surveillance Network. BMC Infect. Dis. 19, 415 (2019).
Bender, B. S. & Small, P. A. Jr. Influenza: pathogenesis and host defense. Semin. Respir. Infect. 7, 38–45 (1992).
Byrd-Leotis, L. et al. Influenza binds phosphorylated glycans from human lung. Sci. Adv. 5, eaav2554 (2019).
Zhang, Z. et al. Structural analyses of Toll-like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 25, 3371–3381.e5 (2018).
Wisskirchen, C., Ludersdorfer, T. H., Muller, D. A., Moritz, E. & Pavlovic, J. The cellular RNA helicase UAP56 is required for prevention of double-stranded RNA formation during influenza A virus infection. J. Virol. 85, 8646–8655 (2011).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Te Velthuis, A. J. W. et al. Mini viral RNAs act as innate immune agonists during influenza virus infection. Nat. Microbiol. 3, 1234–1242 (2018).
Goffic, R. L. et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2, e53 (2006).
Poux, C. et al. A single-stranded oligonucleotide inhibits Toll-like receptor 3 activation and reduces influenza A (H1N1) infection. Front. Immunol. 10, 2161 (2019).
Rappe, J. C. F. et al. A TLR7 antagonist restricts interferon-dependent and -independent immunopathology in a mouse model of severe influenza. J. Exp. Med. 218, e20201631 (2021).
Jansen, A. J. G. et al. Influenza-induced thrombocytopenia is dependent on the subtype and sialoglycan receptor and increases with virus pathogenicity. Blood Adv. 4, 2967–2978 (2020).
Koupenova, M. et al. The role of platelets in mediating a response to human influenza infection. Nat. Commun. 10, 1780 (2019).
Galani, I. E. et al. Interferon-λ mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 46, 875–890.e6 (2017).
Klinkhammer, J. et al. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife 7, e33354 (2018).
Davidson, S., Crotta, S., McCabe, T. M. & Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 5, 3864 (2014).
Major, J. et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369, 712–717 (2020).
Korteweg, C. & Gu, J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am. J. Pathol. 172, 1155–1170 (2008).
Weinheimer, V. K. et al. Influenza A viruses target type II pneumocytes in the human lung. J. Infect. Dis. 206, 1685–1694 (2012).
Stegemann-Koniszewski, S. et al. Alveolar type II epithelial cells contribute to the anti-influenza A virus response in the lung by integrating pathogen- and microenvironment-derived signals. mBio 7, e00276-16 (2016).
Short, K. R. et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 47, 954–966 (2016).
Dunning, J. et al. Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat. Immunol. 19, 625–635 (2018). This paper provides an extensive analysis of acute host responses in adults hospitalized with influenza revealing distinct IFN-related signatures associated with milder disease and neutrophil-dominated inflammatory responses with severe outcomes.
Sidhu, J. K. et al. Delayed mucosal anti-viral responses despite robust peripheral inflammation in fatal COVID-19. J. Infect. Dis. https://doi.org/10.1093/infdis/jiad590 (2023).
Garcia-Sastre, A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 162, 12–18 (2011).
Xia, H. et al. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 33, 108234 (2020).
de Jong, M. D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006).
Wang, Z. et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc. Natl Acad. Sci. USA 111, 769–774 (2014).
Nguyen, T. H. O. et al. Immune cellular networks underlying recovery from influenza virus infection in acute hospitalized patients. Nat. Commun. 12, 2691 (2021). This study characterizes immune cellular networks in longitudinal samples from patients hospitalized with acute influenza, providing a detailed description of factors driving susceptibility, severity and recovery.
Thwaites, R. S. et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci. Immunol. 6, eabg9873 (2021).
Li, H. et al. Internal genes of a highly pathogenic H5N1 influenza virus determine high viral replication in myeloid cells and severe outcome of infection in mice. PLoS Pathog. 14, e1006821 (2018).
Wang, Z. et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8+ T cells. Nat. Commun. 6, 6833 (2015).
Rydyznski Moderbacher, C. et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183, 996–1012.e19 (2020).
Koutsakos, M. et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2, 100208 (2021).
Oshansky, C. M. et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am. J. Respir. Crit. Care Med. 189, 449–462 (2014).
Jenne, C. N. et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 13, 169–180 (2013).
Veras, F. P. et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 217, e20201129 (2020).
Tang, B. M. et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat. Commun. 10, 3422 (2019).
Corry, J. et al. Infiltration of inflammatory macrophages and neutrophils and widespread pyroptosis in lung drive influenza lethality in nonhuman primates. PLoS Pathog. 18, e1010395 (2022).
Zhu, L. et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. J. Infect. Dis. 217, 428–437 (2018).
Lim, K. et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349, aaa4352 (2015).
Cheung, C. Y. et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360, 1831–1837 (2002).
Ferreira, A. C. et al. Severe influenza infection is associated with inflammatory programmed cell death in infected macrophages. Front. Cell Infect. Microbiol. 13, 1067285 (2023).
Yu, W. C. et al. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J. Virol. 85, 6844–6855 (2011).
Wong, S. S. et al. Severe influenza is characterized by prolonged immune activation: results from the SHIVERS cohort study. J. Infect. Dis. 217, 245–256 (2018). This study shows that persistent immune activation characterized by increased monocyte and CD8+ T cell responses is observed in influenza virus-confirmed hospitalized patients with severe acute respiratory illness in line with decreased levels of regulatory mediators and excessive inflammatory cytokines in blood.
Cole, S. L. et al. M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight 2, e91868 (2017).
Smeeth, L. et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 351, 2611–2618 (2004).
Lichenstein, R., Magder, L. S., King, R. E. & King, J. C. Jr. The relationship between influenza outbreaks and acute ischemic heart disease in Maryland residents over a 7-year period. J. Infect. Dis. 206, 821–827 (2012).
Ichiyama, T. et al. Analysis of cytokine levels and NF-kappaB activation in peripheral blood mononuclear cells in influenza virus-associated encephalopathy. Cytokine 27, 31–37 (2004).
Kawada, J. et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J. Infect. Dis. 188, 690–698 (2003).
Gonzalez, B. E. & Brust, D. G. Novel influenza A (H1N1) presenting as an acute febrile encephalopathy in a mother and daughter. Clin. Infect. Dis. 49, 1966–1967 (2009).
Lee, N. et al. Acute encephalopathy associated with influenza A infection in adults. Emerg. Infect. Dis. 16, 139–142 (2010).
Sivadon-Tardy, V. et al. Guillain-Barre syndrome and influenza virus infection. Clin. Infect. Dis. 48, 48–56 (2009).
Tam, C. C., O’Brien, S. J. & Rodrigues, L. C. Influenza, campylobacter and mycoplasma infections, and hospital admissions for Guillain-Barre syndrome, England. Emerg. Infect. Dis. 12, 1880–1887 (2006).
Han, F. et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann. Neurol. 70, 410–417 (2011).
Ayala, E., Kagawa, F. T., Wehner, J. H., Tam, J. & Upadhyay, D. Rhabdomyolysis associated with 2009 influenza A(H1N1). JAMA 302, 1863–1864 (2009).
Everitt, A. R. et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 484, 519–523 (2012).
Zhang, Y. H. et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat. Commun. 4, 1418 (2013).
Pan, Y. et al. IFITM3 Rs12252-C variant increases potential risk for severe influenza virus infection in Chinese population. Front. Cell Infect. Microbiol. 7, 294 (2017).
Lopez-Rodriguez, M. et al. IFITM3 and severe influenza virus infection. No evidence of genetic association. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1811–1817 (2016).
Mills, T. C. et al. IFITM3 and susceptibility to respiratory viral infections in the community. J. Infect. Dis. 209, 1028–1031 (2014).
Randolph, A. G. et al. Evaluation of IFITM3 rs12252 association with severe pediatric influenza infection. J. Infect. Dis. 216, 14–21 (2017).
Allen, E. K. et al. SNP-mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat. Med. 23, 975–983 (2017).
Liu, Y. et al. Genetic variants in IL1A and IL1B contribute to the susceptibility to 2009 pandemic H1N1 influenza A virus. BMC Immunol. 14, 37 (2013).
Garcia-Ramirez, R. A. et al. TNF, IL6, and IL1B polymorphisms are associated with severe influenza A (H1N1) virus infection in the Mexican population. PLoS ONE 10, e0144832 (2015).
Keynan, Y. et al. Chemokine receptor 5 Δ32 allele in patients with severe pandemic (H1N1) 2009. Emerg. Infect. Dis. 16, 1621–1622 (2017).
Zhou, J. et al. A functional variation in CD55 increases the severity of 2009 pandemic H1N1 influenza A virus infection. J. Infect. Dis. 206, 495–503 (2012).
Zuniga, J. et al. Genetic variants associated with severe pneumonia in A/H1N1 influenza infection. Eur. Respir. J. 39, 604–610 (2012).
Chen, Y. et al. Rare variant MX1 alleles increase human susceptibility to zoonotic H7N9 influenza virus. Science 373, 918–922 (2021). This study identifies a multitude of single-nucleotide polymorphisms within the MX1 gene that are associated with increased susceptibility to H7N9 infection and shows these to impact MxA-mediated antiviral activity.
Cheng, Z. et al. Identification of TMPRSS2 as a susceptibility gene for severe 2009 pandemic A(H1N1) influenza and A(H7N9) influenza. J. Infect. Dis. 212, 1214–1221 (2015).
Esposito, S. et al. Toll-like receptor 3 gene polymorphisms and severity of pandemic A/H1N1/2009 influenza in otherwise healthy children. Virol. J. 9, 270 (2012).
Lim, H. K. et al. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J. Exp. Med. 216, 2038–2056 (2019).
Lee, N. et al. IFITM3, TLR3, and CD55 gene SNPs and cumulative genetic risks for severe outcomes in Chinese patients with H7N9/H1N1pdm09 influenza. J. Infect. Dis. 216, 97–104 (2017).
Ciancanelli, M. J. et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 348, 448–453 (2015).
Hernandez, N. et al. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J. Exp. Med. 215, 2567–2585 (2018).
McCullers, J. A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 12, 252–262 (2014).
Brundage, J. F. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 6, 303–312 (2006).
Morens, D. M., Taubenberger, J. K. & Fauci, A. S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198, 962–970 (2008).
Russell, C. D. et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2, e354–e365 (2021).
Wrammert, J. et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453, 667–671 (2008).
Herati, R. S. et al. Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci. Immunol. 2 (2017).
Bentebibel, S. E. et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 5, 176ra132 (2013).
Koutsakos, M. et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 10, eaan8405 (2018). This study describes the role of the antibody axis following influenza vaccination and defines haemagglutinin-specific B cells in different tissues.
Turner, J. S. et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 586, 127–132 (2020).
Thevarajan, I. et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 26, 453–455 (2020).
Andrews, S. F. et al. An influenza H1 hemagglutinin stem-only immunogen elicits a broadly cross-reactive B cell response in humans. Sci. Transl. Med. 15, eade4976 (2023).
Hansen, L. et al. Human anti-N1 monoclonal antibodies elicited by pandemic H1N1 virus infection broadly inhibit HxN1 viruses in vitro and in vivo. Immunity 56, 1927–1938.e8 (2023).
Sutton, H. J. et al. Atypical B cells are part of an alternative lineage of B cells that participates in responses to vaccination and infection in humans. Cell Rep. 34, 108684 (2021).
Nellore, A. et al. A transcriptionally distinct subset of influenza-specific effector memory B cells predicts long-lived antibody responses to vaccination in humans. Immunity 56, 847–863.e8 (2023).
Nguyen, T. H. O. et al. Robust SARS-CoV-2 T cell responses with common TCRαβ motifs toward COVID-19 vaccines in patients with hematological malignancy impacting B cells. Cell Rep. Med. 4, 101017 (2023).
Lartey, S. et al. Live-attenuated influenza vaccine induces tonsillar follicular T helper cell responses that correlate with antibody induction. J. Infect. Dis. 221, 21–32 (2020).
Brenna, E. et al. CD4+ T follicular helper cells in human tonsils and blood are clonally convergent but divergent from non-Tfh CD4+ cells. Cell Rep. 30, 137–152.e5 (2020).
McMichael, A. J., Gotch, F. M., Noble, G. R. & Beare, P. A. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309, 13–17 (1983).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19, 1305–1312 (2013).
Moskophidis, D. & Kioussis, D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188, 223–232 (1998).
Valkenburg, S. A. et al. Molecular basis for universal HLA-A*02:01-restricted CD8+ T-cell immunity against influenza viruses. Proc. Natl Acad. Sci. USA 113, 4440–4445 (2016).
Wagstaffe, H. R. et al. Mucosal and systemic immune correlates of viral control after SARS-CoV-2 infection challenge in seronegative adults. Sci. Immunol. 9, eadj9285 (2024).
Hensen, L. et al. CD8+ T cell landscape in Indigenous and non-Indigenous people restricted by influenza mortality-associated HLA-A*24:02 allomorph. Nat. Commun. 12, 2931 (2021).
van de Sandt, C. E. et al. Challenging immunodominance of influenza-specific CD8+ T cell responses restricted by the risk-associated HLA-A*68:01 allomorph. Nat. Commun. 10, 5579 (2019).
Grant, E. et al. Nucleoprotein of influenza A virus is a major target of immunodominant CD8+ T-cell responses. Immunol. Cell Biol. 91, 184–194 (2013).
Habel, J. R. et al. HLA-A*11:01-restricted CD8+ T cell immunity against influenza A and influenza B viruses in Indigenous and non-Indigenous people. PLoS Pathog. 18, e1010337 (2022).
Bhatt, S., Holmes, E. C. & Pybus, O. G. The genomic rate of molecular adaptation of the human influenza A virus. Mol. Biol. Evol. 28, 2443–2451 (2011).
Quinones-Parra, S. et al. Preexisting CD8+ T cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc. Natl Acad. Sci. USA 111, 1049–1054 (2014).
Koutsakos, M. et al. Human CD8+ T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 20, 613–625 (2019).
Nayak, J. L. et al. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J. Infect. Dis. 207, 297–305 (2012).
Nayak, J. L., Richards, K. A., Yang, H., Treanor, J. J. & Sant, A. J. Effect of influenza A(H5N1) vaccine prepandemic priming on CD4+ T-cell responses. J. Infect. Dis. 211, 1408–1417 (2014).
Alam, S., Knowlden, Z. A. G., Sangster, M. Y. & Sant, A. J. CD4 T cell help is limiting and selective during the primary B cell response to influenza virus infection. J. Virol. 88, 314–324 (2014).
Cohen, K. W. et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2, 100354 (2021).
Taus, E. et al. Dominant CD8+ T cell nucleocapsid targeting in SARS-CoV-2 infection and broad spike targeting from vaccination. Front. Immunol. 13, 835830 (2022).
Mudd, P. A. et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 185, 603–613.e15 (2022).
Vita, R. et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2019).
McKinney, D. M. et al. A strategy to determine HLA class II restriction broadly covering the DR, DP, and DQ allelic variants most commonly expressed in the general population. Immunogenetics 65, 357–370 (2013).
Hertz, T. et al. HLA targeting efficiency correlates with human T-cell response magnitude and with mortality from influenza A infection. Proc. Natl Acad. Sci. USA 110, 13492–13497 (2013).
Falfán-Valencia, R. et al. An increased frequency in HLA class I alleles and haplotypes suggests genetic susceptibility to influenza A (H1N1) 2009 pandemic: a case-control study. J. Immunol. Res. 2018, 3174868 (2018).
Augusto, D. G. et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection. Nature 620, 128–136 (2023).
Peng, Y. et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 21, 1336–1345 (2020).
Hillaire, M. L. et al. Characterization of the human CD8+ T cell response following infection with 2009 pandemic influenza H1N1 virus. J. Virol. 85, 12057–12061 (2011).
Sant, S. et al. Single-cell approach to influenza-specific CD8+ T cell receptor repertoires across different age groups, tissues, and following influenza virus infection. Front. Immunol. 9, 1453 (2018).
Rowntree, L. C. et al. SARS-CoV-2-specific T cell memory with common TCRαβ motifs is established in unvaccinated children who seroconvert after infection. Immunity 55, 1299–1315.e4 (2022).
van de Sandt, C. E. et al. Human influenza A virus-specific CD8+ T-cell response is long-lived. J. Infect. Dis. 212, 81–85 (2015).
Mettelman, R. C. et al. Baseline innate and T cell populations are correlates of protection against symptomatic influenza virus infection independent of serology. Nat. Immunol. 24, 1511–1526 (2023).
Pizzolla, A. et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 128, 721–733 (2018).
Pizzolla, A. et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2, eaam6970 (2017).
Messaoudi, I., Guevara Patiño, J. A., Dyall, R., LeMaoult, J. & Nikolich-Zugich, J. Direct link between MHC polymorphism, T cell avidity, and diversity in immune defense. Science 298, 1797–1800 (2002).
Ndhlovu, Z. M. et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity 43, 591–604 (2015).
Wang, Z. et al. Clonally diverse CD38+HLA-DR+CD8+ T cells persist during fatal H7N9 disease. Nat. Commun. 9, 824 (2018).
van de Sandt, C. E. et al. Newborn and child-like molecular signatures in older adults stem from TCR shifts across human lifespan. Nat. Immunol. 24, 1890–1907 (2023). This study shows a detailed examination of influenza virus-specific CD8+ T cell immunity across the human lifespan, identifying age-related changes in the T cell repertoire with implications for sustaining optimal immunity.
Jia, X. et al. High expression of CD38 and MHC class II on CD8+ T cells during severe influenza disease reflects bystander activation and trogocytosis. Clin. Transl. Immunol. 10, e1336 (2021).
Paterson, S. et al. Innate-like gene expression of lung-resident memory CD8+ T cells during experimental human influenza: a clinical study. Am. J. Respir. Crit. Care Med. 204, 826–841 (2021). This study provides a transcriptomic analysis of blood and lung CD8+ T cells after experimental influenza virus infection, identifying innate-like functions of CD8+ TRM cells; it also associates pre-existing blood influenza virus-specific CD8+ T cell frequency with lower viral shedding.
Sherman, A. C., Mehta, A., Dickert, N. W., Anderson, E. J. & Rouphael, N. The future of flu: a review of the human challenge model and systems biology for advancement of influenza vaccinology. Front. Cell Infect. Microbiol. 9, 107 (2019).
Carrat, F. et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am. J. Epidemiol. 167, 775–785 (2008). This study provides a meta-analysis of more than 1,000 volunteers experimentally infected with influenza virus, defining viral and symptom kinetics across viral strains.
Chiu, C., Ellebedy, A. H., Wrammert, J. & Ahmed, R. B cell responses to influenza infection and vaccination. Curr. Top. Microbiol. Immunol. 386, 381–398 (2015).
Hobson, D., Curry, R. L., Beare, A. S. & Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 70, 767–777 (1972).
Gould, V. M. W. et al. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front. Microbiol. 8, 900 (2017).
Park, J. K. et al. Evaluation of preexisting anti-hemagglutinin stalk antibody as a correlate of protection in a healthy volunteer challenge with influenza A/H1N1pdm virus. mBio 9, e02284-17 (2018).
Han, A. et al. Safety and efficacy of CR6261 in an influenza A H1N1 healthy human challenge model. Clin. Infect. Dis. 73, e4260–e4268 (2021).
Wilkinson, T. M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Pleguezuelos, O. et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. NPJ Vaccines 5, 22 (2020).
Francis, T., Pearson, H. E., Salk, J. E. & Brown, P. N. Immunity in human subjects artificially infected with influenza virus, type B. Am. J. Public Health Nations Health 34, 317–334 (1944).
Memoli, M. J. et al. Influenza A reinfection in sequential human challenge: implications for protective immunity and “universal” vaccine development. Clin. Infect. Dis. 70, 748–753 (2020).
La Gruta, N. L., Kedzierska, K., Stambas, J. & Doherty, P. C. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 85, 85–92 (2007).
Woods, C. W. et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS ONE 8, e52198 (2013).
Sobel Leonard, A. et al. Deep sequencing of influenza A virus from a human challenge study reveals a selective bottleneck and only limited intrahost genetic diversification. J. Virol. 90, 11247–11258 (2016).
Killingley, B. et al. Use of a human influenza challenge model to assess person-to-person transmission: proof-of-concept study. J. Infect. Dis. 205, 35–43 (2012). This study develops the influenza human challenge model to study viral transmission.
Nguyen-Van-Tam, J. S. et al. Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment. PLoS Pathog. 16, e1008704 (2020).
Bueno de Mesquita, P. J., Noakes, C. J. & Milton, D. K. Quantitative aerobiologic analysis of an influenza human challenge-transmission trial. Indoor Air 30, 1189–1198 (2020).
Bell, J. A. et al. Artificially induced Asian influenza in vaccinated and unvaccinated volunteers. J. Am. Med. Assoc. 165, 1366–1373 (1957).
Balasingam, S. & Wilder-Smith, A. Randomized controlled trials for influenza drugs and vaccines: a review of controlled human infection studies. Int. J. Infect. Dis. 49, 18–29 (2016).
Cox, R. J. Correlates of protection to influenza virus, where do we go from here? Hum. Vaccin. Immunother. 9, 405–408 (2013).
Centers for Disease Control and Prevention (CDC). Fluzone high-dose seasonal influenza vaccine. Centers for Disease Control and Prevention https://www.cdc.gov/flu/prevent/qa_fluzone.htm (2023).
Yager, E. J. et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723 (2008).
Goodwin, K., Viboud, C. & Simonsen, L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24, 1159–1169 (2006).
Zhang, Q., Bastard, P., Effort, C. H. G., Cobat, A. & Casanova, J. L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 603, 587–598 (2022).
Zhang, Q. et al. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J. Exp. Med. 219, e20220514 (2022).
Van Kerkhove, M. D. et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 8, e1001053 (2011).
Prasad, N. et al. Influenza-associated outcomes among pregnant, postpartum, and nonpregnant women of reproductive age. J. Infect. Dis. 219, 1893–1903 (2019).
Yates, L. et al. Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant. Health Technol. Assess. 14, 109–182 (2010).
Håberg, S. E. et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N. Engl. J. Med. 368, 333–340 (2013).
Le Gars, M. et al. Increased proinflammatory responses of monocytes and plasmacytoid dendritic cells to influenza A virus infection during pregnancy. J. Infect. Dis. 214, 1666–1671 (2016).
Vanders, R. L., Gibson, P. G., Murphy, V. E. & Wark, P. A. Plasmacytoid dendritic cells and CD8 T cells from pregnant women show altered phenotype and function following H1N1/09 infection. J. Infect. Dis. 208, 1062–1070 (2013).
Savic, M. et al. Distinct T and NK cell populations may serve as immune correlates of protection against symptomatic pandemic influenza A(H1N1) virus infection during pregnancy. PLoS ONE 12, e0188055 (2017).
Le Gars, M. et al. Pregnancy-induced alterations in NK cell phenotype and function. Front. Immunol. 10, 2469 (2019).
Kay, A. W. et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc. Natl Acad. Sci. USA 111, 14506–14511 (2014).
Allotey, J. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370, m3320 (2020).
Ferrara, A. et al. Perinatal complications in individuals in California with or without SARS-CoV-2 infection during pregnancy. JAMA Intern. Med. 182, 503–512 (2022).
Habel, J. R. et al. Immune profiling of SARS-CoV-2 infection during pregnancy reveals NK cell and γδ T cell perturbations. JCI Insight 8, e167157 (2023).
Flint, S. M. et al. Disproportionate impact of pandemic (H1N1) 2009 influenza on Indigenous people in the top end of Australia’s Northern Territory. Med. J. Aust. 192, 617–622 (2010).
Li-Kim-Moy, J. et al. Australian vaccine preventable disease epidemiological review series: influenza 2006 to 2015. Commun. Dis. Intell. Q. Rep. 40, E482–e495 (2016).
Betts, J. M. et al. Influenza-associated hospitalisation and mortality rates among global Indigenous populations; a systematic review and meta-analysis. PLoS Glob. Public Health 3, e0001294 (2023).
Duncan, C. J. A. et al. Life-threatening viral disease in a novel form of autosomal recessive IFNAR2 deficiency in the Arctic. J. Exp. Med. 219, e20212427 (2022).
Hensen, L. et al. Robust and prototypical immune responses toward influenza vaccines in the high-risk group of Indigenous Australians. Proc. Natl Acad. Sci. USA 118, e2109388118 (2021).
Zhang, W. et al. Robust and prototypical immune responses toward COVID-19 vaccine in First Nations peoples are impacted by comorbidities. Nat. Immunol. 24, 966–978 (2023).
Kochanek, K. D., Murphy, S. L., Xu, J. & Arias, E. Deaths: final data for 2017. Natl Vital Stat. Rep. 68, 9 (2019).
Qin, R. et al. Prevaccination glycan markers of response to an influenza vaccine implicate the complement pathway. J. Proteome Res. 21, 1974–1985 (2022).
Hulme, K. D., Noye, E. C., Short, K. R. & Labzin, L. I. Dysregulated inflammation during obesity: driving disease severity in influenza virus and SARS-CoV-2 infections. Front. Immunol. 12, 770066 (2021).
Neidich, S. D. et al. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 41, 1324–1330 (2017).
Honce, R. & Schultz-Cherry, S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 10, 1071 (2019).
Tong, M. Z. et al. Elevated BMI reduces the humoral response to SARS-CoV-2 infection. Clin. Transl. Immunol. 12, e1476 (2023).
Near, A. M., Tse, J., Young-Xu, Y., Hong, D. K. & Reyes, C. M. Burden of influenza hospitalization among high-risk groups in the United States. BMC Health Serv. Res. 22, 1209 (2022).
Zhang, W. et al. Robust immunity to influenza vaccination in haematopoietic stem cell transplant recipients following reconstitution of humoral and adaptive immunity. Clin. Transl. Immunol. 12, e1456 (2023).
Künzli, M. et al. Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells. Sci. Immunol. 7, eadd3075 (2022).
Pardi, N. et al. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat. Commun. 13, 4677 (2022).
van de Ven, K. et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 8, eadc9937 (2022).
Arevalo, C. P. et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 378, 899–904 (2022).
Jorgensen, S. E. et al. Defective RNA sensing by RIG-I in severe influenza virus infection. Clin. Exp. Immunol. 192, 366–376 (2018).
Bravo Garcia-Morato, M. et al. Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J. Allergy Clin. Immunol. 144, 309–312.e10 (2019).
Prabhu, S. S., Chakraborty, T. T., Kumar, N. & Banerjee, I. Association between IFITM3 rs12252 polymorphism and influenza susceptibility and severity: a meta-analysis. Gene 674, 70–79 (2018).
Xuan, Y. et al. IFITM3 rs12252 T>C polymorphism is associated with the risk of severe influenza: a meta-analysis. Epidemiol. Infect. 143, 2975–2984 (2015).
Rogo, L. D. et al. Seasonal influenza A/H3N2 virus infection and IL-1β, IL-10, IL-17, and IL-28 polymorphisms in Iranian population. J. Med. Virol. 88, 2078–2084 (2016).
Maestri, A. et al. Siaα2-3Galβ1- receptor genetic variants are associated with influenza A(H1N1)pdm09 severity. PLoS ONE 10, e0139681 (2015).
Habibi, M. S. et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 191, 1040–1049 (2015).
Bartsch, Y. C. et al. Antibody effector functions are associated with protection from respiratory syncytial virus. Cell 185, 4873–4886.e10 (2022).
Habibi, M. S. et al. Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science 370, eaba9301 (2020).
Jozwik, A. et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 6, 10224 (2015).
Tyrrell, D. A. A view from the common cold unit. Antivir. Res. 18, 105–125 (1992).
Coultas, J. A., Cafferkey, J., Mallia, P. & Johnston, S. L. Experimental antiviral therapeutic studies for human rhinovirus infections. J. Exp. Pharmacol. 13, 645–659 (2021).
Hansel, T. T. et al. A comprehensive evaluation of nasal and bronchial cytokines and chemokines following experimental rhinovirus infection in allergic asthma: increased interferons (IFN-γ and IFN-λ) and type 2 inflammation (IL-5 and IL-13). EBioMedicine 19, 128–138 (2017).
Mallia, P. et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 183, 734–742 (2011).
Killingley, B. et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 28, 1031–1041 (2022).
Acknowledgements
K.K. is a recipient of a National Health and Medical Research Council (NHMRC) L1 Fellowship (1173871). T.H.O.N. and L.C.R. are recipients of a NHMRC EL1 Fellowship (1194036, 2026357). K.K., T.H.O.N. and L.C.R. are supported by NHMRC MRFF Award (2016062). B.Y.C. is supported by NHMRC Ideas Grants (2001346 and 2019097). R.S.T. is supported by the National Institute for Health and Care Research (NIHR) Imperial Biomedical Research Centre (BRC; NIHR Imperial BRC grant P70668), the Health Protection Research Unit (HPRU) in Respiratory Infections at Imperial College London in partnership with Public Health England/UK Health Security Agency and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UH2AI176172. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
T.H.O.N., L.C.R. and B.Y.C. contributed equally to all aspects of the article. All authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Andreas Wack and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T.H.O., Rowntree, L.C., Chua, B.Y. et al. Defining the balance between optimal immunity and immunopathology in influenza virus infection. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01029-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01029-1