Abstract
Background
Congenital heart disease (CHD) are the most common malformations from birth. The severity of the different forms of CHD varies extensively from superficial mild lesions with follow-up for decades without any treatment to complex cyanotic malformations requiring urgent surgical intervention. microRNAs have been found to be crucial in cardiac development, giving rise to possible phenotypes in CHD.
Objectives
We aimed to evaluate the expression of miRNAs in 86 children with CHD and divided into cyanotic and non-cyanotic heart defects and 110 controls.
Methods
The miRNAs expression of miR-21-5p, miR-155-5p, miR-221-3p, miR-26a-5p, and miR-144-3p were analyzed by RT-qPCR. In addition, the expressions of the miRNAs studied were correlated with the clinical characteristics of both the children and the mothers.
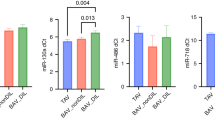
Results
The expression levels of miR-21-5-5p, miR-15-5p5, miR-221-3p, and miR-26-5p significantly differed between CHD and control subjects. Moreover, miR-21-5p levels were higher in patients with cyanotic versus non-cyanotic CHD patients.
Conclusion
The expression levels of miRNAs of pediatric patients with CHD could participating in the development of cardiac malformations. Additionally, the high expression of miR-21-5p in cyanotic CHD children may be related to greater severity of illness relative to non-cyanotic CHD.
Impact
-
This study adds to knowledge of the association between microRNAs and congenital heart disease in children.
-
The expression levels of miR-21-5-5p, miR-15-5p5, miR-221-3p, and miR-26-5p of pediatric patients with CHD could be involved in the development and phenotype present in pediatric patients.
-
miR-21-5p may help to discriminate between cyanotic and non-cyanotic CHD.
-
In the future, the miRNAs studied could have applications as clinical biomarkers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data and materials are available for transparency. All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Marquez-González, H., Yañez-Gutierrez, L., Rivera-May, J., Lopez-Gallegos, D. & Gutierrez, E. Análisis Demográfico de Una Clínica de Cardiopatías Congénitas Del Instituto Mexicano Del Seguro Social, Con Interés En El Adulto. Arch. Cardiol. Méx. 88, 360–368 (2018).
Smith, T., Rajakaruna, C., Caputo, M. & Emanueli, C. MicroRNAs in congenital heart disease. Ann. Transl. Med. 3, 333 (2015).
Claeys, M. J., Bondue, A., Lancellotti, P. & De Pauw, M. Summary of 2020 ESC guidelines on non-STE ACS, adult congenital heart disease, sports cardiology and atrial fibrillation. Acta Cardiol. 77, 864–872 (2022).
Saliba, A. et al. Genetic and genomics in congenital heart disease: a clinical review. J. Pediatr. 96, 279–288 (2019).
Rohit, M. & Shrivastava, S. Acyanotic and cyanotic congenital heart diseases. Indian J. Pediatr. 85, 454–460 (2018).
Quien, M., Ryzhkov, I., Miras, L. & Zarich, S. Complex congenital heart disease: gerbode defect with a bicuspid aortic valve and coarctation of the aorta. CASE 7, 242–244 (2023).
Lim, T. B., Foo, S. Y. R. & Chen, C. K. The role of epigenetics in congenital heart disease. Genes 12, 390 (2021).
Kalayinia, S., Arjmand, F., Maleki, M., Malakootian, M. & Singh, C. P. MicroRNAs: roles in cardiovascular development and disease. Cardiovasc. Pathol. 50, 107296 (2021).
Saliminejad, K., Khorram-Khorshid, H. R., Soleymani-Fard, S. & Ghaffari, S. H. An overview of MicroRNAs: biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 234, 5451–5465 (2019).
Eddy, A. A. The TGF-β route to renal fibrosis is not linear: the MiR-21 Viaduct. J. Am. Soc. Nephrol. 22, 1573–1575 (2011).
Yuan, X. et al. MiR-144-3p enhances cardiac fibrosis after myocardial infarction by targeting PTEN. Front. Cell Dev. Biol. 7, 249 (2019).
Icli, B., Dorbala, P. & Feinberg, M. W. An emerging role for the MiR-26 family in cardiovascular disease. Trends Cardiovasc. Med. 24, 241–248 (2014).
Wu, X. G. et al. Cancer-derived exosomal MiR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 22, 397–410 (2019).
Clement, M., Viggiani, G., Chen, Y. W., Coulis, G. & Castaldi, A. MicroRNA and ROS Crosstalk in Cardiac and Pulmonary Diseases. Int. J. Mol. Sci. 21, 4370 (2020).
Wang, G., Wang, B. & Yang, P. Epigenetics in congenital heart disease. J. Am. Heart Assoc. 11, e025163 (2022).
Zhu, S. et al. Identification of maternal serum microRNAs as novel non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Clin. Chim. Acta 424, 66–72 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408 (2001).
Kumaraswamy, R., Volkmann, I. & Thum, T. Regulation and function of MiRNA-21 in health and disease. RNA Biol. 8, 706–713 (2011).
Li, M. et al. MiR-1-3p that correlates with left ventricular function of HCM can serve as a potential target and differentiate HCM from DCM. J. Transl. Med. 16, 161 (2018).
Rubis, P. et al. Relations between circulating micrornas (Mir-21, Mir-26, Mir-29, Mir-30 And Mir-133a), extracellular matrix fibrosis and serum markers of fibrosis in dilated cardiomyopathy. Int. J. Cardiol. 231, 201–206 (2017).
Liu, S. et al. Micro-RNA 21targets dual specific phosphatase 8 to promote collagen synthesis in high glucose treated primary cardiac fibroblasts. Can. J. Cardiol. 30, 1689–1699 (2014).
Xiao, J. et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal Mir-21 by targeting PDCD4. Cell Death Dis. 7, e2277 (2016).
Liu, Y. et al. A feedback regulatory loop between HIF-1 Alpha and Mir-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 588, 3137–3146 (2014).
Zlabinger, K. et al. MiR-21, MiR-29a, GATA4, and MEF2c expression changes in Endothelin-1 and Angiotensin II cardiac hypertrophy stimulated Isl-1(+) Sca-1(+) c-kit(+) porcine cardiac progenitor cells in vitro. Cells 8, 1416 (2019).
Cheng, Y. et al. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J. Mol. Cell Cardiol. 47, 5–14 (2009).
Xu, H. F. et al. MicroRNA21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol. Med. Rep. 10, 161–168 (2014).
Sayed, D. et al. MicroRNA-21 Targets Sprouty2 and promotes cellular outgrowths. Mol. Biol. Cell 19, 3272–3282 (2008).
Li, Q. et al. Inhibition of miR-21 alleviated cardiac perivascular fibrosis via repressing EndMT in T1DM. J. Cell Mol. Med. 24, 910–920 (2020).
Sanford, L. P. et al. TGF beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGF beta knockout phenotypes. Development 124, 2659–2670 (1997).
Bartram, U. et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-Beta (2)- knockout mice. Circulation 103, 2745–2752 (2001).
Jiao, K. et al. TGF beta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 133, 4585–4593 (2006).
Sridurongrit, S. et al. Signaling via the TGF beta Type I Receptor Alk5 in heart development. Dev. Biol. 322, 208–218 (2008).
Chuppa, S. et al. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in cardiorenal syndrome Type 4. Kidney Int. 93, 375–389 (2018).
Shen, H. et al. miR-21 enhances the protective effect of loperamide on rat cardiomyocytes against hypoxia/reoxygenation, reactive oxygen species production and apoptosis via regulating Akap8 and Bard1 expression. Exp. Ther. Med. 17, 1312–1320 (2019).
Zhou, X. L. et al. miR-21 promotes cardiac fibroblast-to-myofibroblast transformation and myocardial fibrosis by targeting Jagged1. J. Cell Mol. Med. 22, 3816–3824 (2018).
Bang, C. et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Investig. 124, 2136–2146 (2014).
Kang, Z. et al. Remote ischemic preconditioning upregulates MicroRNA-21 to protect the kidney in children with congenital heart disease undergoing cardiopulmonary bypass. Pediatr. Nephrol. 33, 911–919 (2018).
Lacedonia, D. et al. MicroRNA expression profile during different conditions of hypoxia. Oncotarget 9, 35114–35122 (2018).
Wang, J. et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt Pathways. J. Nanobiotechnol. 19, 1–13 (2021).
Li, C., Fei, K., Tian, F., Gao, C. & Song, Y. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating MiR-21-3p/MAT2B signaling transduction. Croat. Med. 60, 439 (2019).
Jiang, Y. et al. Exosomes secreted by HUVECs attenuate hypoxia/reoxygenation-induced apoptosis in neural cells by suppressing MiR-21-3p. Am. J. Transl. Res. 10, 3529 (2018).
Peñaloza, E., Soto-Carrasco, G. & Krause, B. J. MiR-21-5p directly contributes to regulating eNOS expression in human artery endothelial cells under normoxia and hypoxia. Biochem. Pharm. 182, 114288 (2020).
Chen, L., Zheng, S. Y., Yang, C. Q., Ma, B. M. & Jiang, D. Mir-155-5p inhibits the proliferation and migration of VSMCs and HUVECs in atherosclerosis by targeting ATK1. Eur. Rev. Med. Pharm. Sci. 23, 2223 (2019).
Zhao, J. & Zeng, Z. Combined effects of AKT Serine/Threonine Kinase 1 polymorphisms and environment on congenital heart disease risk: a case-control study. Medicine 99, e20400 (2020).
Chang, Z. et al. Deletion of Akt1 causes heart defects and abnormal cardiomyocyte proliferation. Dev. Biol. 347, 384–391 (2010).
Zhao, D. et al. Integrative bioinformatics analysis revealed mitochondrial defects underlying hypoplastic left heart syndrome. Int. J. Gen. Med. 14, 9747–9760 (2021).
Gordon, J. W., Shaw, J. A. & Kirshenbaum, L. A. Multiple facets of NF-ΚB in the Heart: To Be or Not to NF-ΚB. Circ. Res. 108, 1122–1132 (2011).
Zhou, X. et al. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: a MiRNA expression analysis. Gene 673, 181–193 (2018).
Cordenonsi, M. et al. Links between tumor suppressors: P53 is required for TGF-β gene responses by cooperating with smads. Cell 113, 301–314 (2003).
Hanna, A., Humeres, C. & Frangogiannis, N. G. The role of smad signaling cascades in cardiac fibrosis. Cell Signal. 77, 109826 (2021).
Lu, M., Qin, Q., Yao, J., Sun, L. & Qin, X. Induction of LOX by TGF-Β1/Smad/AP-1 signaling aggravates rat myocardial fibrosis and heart failure. IUBMB Life 71, 1729–1739 (2019).
Zhong, L. et al. The TBX1/MiR-193a-3p/TGF-Β2 axis mediates CHD by promoting ferroptosis. Oxid. Med. Cell Longev. 7, 1–13 (2022).
Telkoparan-Akillilar, P., Cevik, D. & Yilmaz, A. Expression patterns of MiR-34a, MiR-125b, MiR-221 and antioxidant gene NRF2 in plasma samples of patients with atherosclerosis. J. Biosci. 47, 1 (2022).
Zhang, Z. H. et al. MicroRNA-26 was decreased in rat cardiac hypertrophy model and may be a promising therapeutic target. J. Cardiovasc. Pharm. 62, 312–319 (2013).
Monteiro da Rocha, A., Ding, J., Slawny, N., Wolf, A. M. & Smith, G. D. Loss of glycogen synthase kinase 3 isoforms during murine oocyte growth induces offspring cardiac dysfunction. Biol. Reprod. 92, 127 (2015).
Luo, X. et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J. Clin. Investig. 123, 1939–1951 (2013).
Ding, H. et al. Combined detection of MiR-21-5p, MiR-30a-3p, MiR-30a-5p, MiR-155-5p, MiR-216a, and MiR-217 for early heart failure diseases screening. Biosci. Rep. 40, 3 (2020).
Jenike, A. E. & Halushka, M. K. MiR-21: a non-specific biomarker of all maladies. Biomark. Res. 9, 18 (2021).
Surina, S. et al. MiR-21 in human cardiomyopathies. Front. Cardiovasc. Med. 8, 767064 (2021).
Acknowledgements
We thank the patients and relatives for collaborating on this project. This work was financed by the budget granted to the Department of Physiology by the National Institute of Cardiology Ignacio Chavez.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the conception, design, acquisition of information, and writing for this commentary. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted by the Declaration of Helsinki and approved by the locally appointed Ethics Committee, Institutional Review Board, Instituto Nacional de Cardiologia Ignacio Chavez, and Instituto Nacional de Perinatologia: Number: 20-1181.
Informed consent
Informed consent was obtained from the parents of participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González-Moyotl, N., Huesca-Gómez, C., Torres-Paz, Y.E. et al. Paediatrics congenital heart disease is associated with plasma miRNAs. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03230-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03230-3