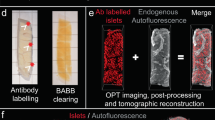
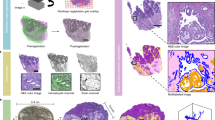
Abstract
A convenient technology to quantify three-dimensional (3D) morphological features would have widespread applications in biomedical research. Based on combined improvements in sample preparation, tomographic imaging and computational processing, we present a procedure for high-resolution 3D quantification of structures within intact adult mouse organs. Using the nonobese diabetic (NOD) mouse model, we demonstrate a correlation between total islet β-cell volume and the onset of type-1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Skau, M., Pakkenberg, B., Buschard, K. & Bock, T. Diabetes 50, 1763–1770 (2001).
Bouwens, L. & Rooman, I. Physiol. Rev. 85, 1255–1270 (2005).
Sharpe, J. et al. Science 296, 541–545 (2002).
Dowson, J.H. Acta Neuropathol. (Berl.) 61, 196–200 (1983).
Dent, J.A., Polson, A.G. & Klymkowsky, M.W. Development 105, 61–74 (1989).
Clancy, B. & Cauller, L.J. J. Neurosci. Methods 83, 97–102 (1998).
Beisker, W., Dolbeare, F. & Gray, J.W. Cytometry 8, 235–239 (1987).
Schnell, S.A., Staines, W.A. & Wessendorf, M.W. J. Histochem. Cytochem. 47, 719–730 (1999).
Neumann, M. & Gabel, D. J. Histochem. Cytochem. 50, 437–439 (2002).
Van de Lest, C.H., Versteeg, E.M., Veerkamp, J.H. & Van Kuppevelt, T.H. J. Histochem. Cytochem. 43, 727–730 (1995).
Cowen, T., Haven, A.J. & Burnstock, G. Histochemistry 82, 205–208 (1985).
Csallany, A.S. & Ayaz, K.L. Lipids 11, 412–417 (1976).
Sillitoe, R.V. & Hawkes, R. J. Histochem. Cytochem. 50, 235–244 (2002).
Bock, T., Pakkenberg, B. & Buschard, K. Diabetes 54, 133–137 (2005).
Tsai, E.B., Sherry, N.A., Palmer, J.P. & Herold, K.C. Diabetologia 49, 261–270 (2006).
Acknowledgements
We thank T. Bock and B. Hellman for helpful discussions. This work was supported by the Medical Research Council (H.M, J.S), the Wenner-Gren foundation (C.E.L), Novo Nordisk Fonden, the Swedish Diabetes Association (D.H), the Juvenile Diabetes Research Foundation (D.H, J.S, U.A), the Swedish Research Council (D.H, U.A) and the Kempe Foundation (U.A.).
Author information
Authors and Affiliations
Contributions
T.A. developed the immunohistochemical protocol and performed immmunohistochemical analyses; A.A. performed the OPT and confocal scanning; H.M. performed OPT reconstructions and islet quantifications; C.E.L performed histological integrity controls; D.H. designed the NOD experimental setup; J.S. developed the subtraction script and supervised the project; U.A. initiated and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
J.S. is the inventor on four patents relating to OPT technology.
Supplementary information
Supplementary Fig. 1
Optimized labeling allows visualization of large-scale specimen.
Supplementary Fig. 2
Example of variation in islet composition for a non-diabetic versus an overtly diabetic NOD mouse.
Supplementary Fig. 3
3D reconstructions of an intact mouse brain labeled for neuronal marker Isl1.
Supplementary Video 1
Iso-surface reconstruction of an adult wild type splenic pancreas. Movie showing a 3D OPT image of an intact splenic mouse pancreas (8 weeks). The reconstruction of the pancreas outline is based on the autofluorescence signal (grey) whereas the insulin expressing cells of the islets (red) are based on the signal from insulin specific antibodies. The specimen is ∼ 1.2 × 0.7 cm.
Rights and permissions
About this article
Cite this article
Alanentalo, T., Asayesh, A., Morrison, H. et al. Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat Methods 4, 31–33 (2007). https://doi.org/10.1038/nmeth985
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth985
This article is cited by
-
Novel Radiotracers for Molecular Imaging of Myocardial Inflammation: an Update Focused on Clinical Translation of Non-18F-FDG Radiotracers
Current Cardiovascular Imaging Reports (2023)
-
Quantitative 3D OPT and LSFM datasets of pancreata from mice with streptozotocin-induced diabetes
Scientific Data (2022)
-
Endometrial receptivity and implantation require uterine BMP signaling through an ACVR2A-SMAD1/SMAD5 axis
Nature Communications (2021)
-
3D imaging of human organs with micrometer resolution - applied to the endocrine pancreas
Communications Biology (2021)
-
Topologically selective islet vulnerability and self-sustained downregulation of markers for β-cell maturity in streptozotocin-induced diabetes
Communications Biology (2020)