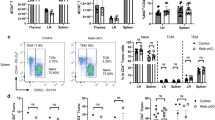
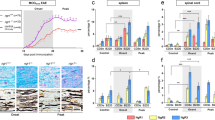
Abstract
It is unclear whether TGF-β, a critical differentiation factor for T cells producing interleukin 17 (TH-17 cells), is required for the initiation of experimental autoimmune encephalomyelitis (EAE) in vivo. Here we show that mice whose T cells cannot respond to TGF-β signaling lack TH-17 cells and do not develop EAE despite the presence of T helper cell type 1 infiltrates in the spinal cord. Local but not systemic antibody blockade of TGF-β prevented TH-17 cell differentiation and the onset of EAE. The pathogen stimulus zymosan, like mycobacterium, induced TH-17 cells and initiated EAE, but the disease was transient and correlated with reduced production of interleukin 23. These data show that TGF-β is essential for the initiation of EAE and suggest that disease progression may require ongoing chronic inflammation and production of interleukin 23.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Billiau, A. & Matthys, P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 70, 849–860 (2001).
Ferber, I.A. et al. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156, 5–7 (1996).
Willenborg, D.O., Fordham, S.A., Staykova, M.A., Ramshaw, I.A. & Cowden, W.B. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J. Immunol. 163, 5278–5286 (1999).
Becher, B., Durell, B.G. & Noelle, R.J. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110, 493–497 (2002).
Gran, B. et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 169, 7104–7110 (2002).
Zhang, G.X. et al. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-β2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J. Immunol. 170, 2153–2160 (2003).
Gutcher, I., Urich, E., Wolter, K., Prinz, M. & Becher, B. Interleukin 18-dependent engagement of interleukin 18 receptor-α is required for autoimmune inflammation. Nat. Immunol. 7, 946–953 (2006).
Harrington, L.E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 (2003).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Gorelik, L. & Flavell, R.A. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 (2000).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Infante-Duarte, C., Horton, H.F., Byrne, M.C. & Kamradt, T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 165, 6107–6115 (2000).
Khader, S.A. et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 175, 788–795 (2005).
Massague, J. The transforming growth factor-β family. Annu. Rev. Cell Biol. 6, 597–641 (1990).
Wahl, S.M., Allen, J.B., Costa, G.L., Wong, H.L. & Dasch, J.R. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor β. J. Exp. Med. 177, 225–230 (1993).
Wahl, S.M. Transforming growth factor β: the good, the bad, and the ugly. J. Exp. Med. 180, 1587–1590 (1994).
Zhang, X. et al. Recovery from experimental allergic encephalomyelitis is TGF-β dependent and associated with increases in CD4+LAP+ and CD4+CD25+ T cells. Int. Immunol. 18, 495–503 (2006).
Szczepanik, M., Tutaj, M., Bryniarski, K. & Dittel, B.N. Epicutaneously induced TGF-β-dependent tolerance inhibits experimental autoimmune encephalomyelitis. J. Neuroimmunol. 164, 105–114 (2005).
Murano, M. et al. Latent TGF-β1-transduced CD4+ T cells suppress the progression of allergic encephalomyelitis. J. Leukoc. Biol. 79, 140–146 (2006).
Cautain, B. et al. Essential role of TGF-β in the natural resistance to experimental allergic encephalomyelitis in rats. Eur. J. Immunol. 31, 1132–1140 (2001).
Fahlen, L. et al. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 737–746 (2005).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 (2005).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Chen, Y. et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326 (2006).
Fontenot, J.D., Rasmussen, J.P., Gavin, M.A. & Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Zamvil, S. et al. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature 317, 355–358 (1985).
Martin, R., McFarland, H.F. & McFarlin, D.E. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 10, 153–187 (1992).
Smith, S.B. & Waksman, B.H. Passive transfer and labelling studies on the cell infiltrate in experimental allergic encephalomyelitis. J. Pathol. 99, 237–244 (1969).
Lafaille, J.J. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 9, 139–151 (1998).
Samoilova, E.B., Horton, J.L., Hilliard, B., Liu, T.S. & Chen, Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161, 6480–6486 (1998).
Gijbels, K., Brocke, S., Abrams, J.S. & Steinman, L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1, 795–805 (1995).
Fossiez, F. et al. Interleukin-17. Int. Rev. Immunol. 16, 541–551 (1998).
Powell, M.B. et al. Lymphotoxin and tumor necrosis factor-α production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int. Immunol. 2, 539–544 (1990).
Baker, D. et al. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur. J. Immunol. 24, 2040–2048 (1994).
Jovanovic, D.V. et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 160, 3513–3521 (1998).
Chabas, D. et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294, 1731–1735 (2001).
Jansson, M., Panoutsakopoulou, V., Baker, J., Klein, L. & Cantor, H. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J. Immunol. 168, 2096–2099 (2002).
Ashkar, S. et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287, 860–864 (2000).
Abel, B., Freigang, S., Bachmann, M.F., Boschert, U. & Kopf, M. Osteopontin is not required for the development of Th1 responses and viral immunity. J. Immunol. 175, 6006–6013 (2005).
da Silva, A.P. et al. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-α expression and non-programmed cell death. J. Cell. Physiol. 208, 629–639 (2006).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508 (2002).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Komiyama, Y. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
McKenzie, B.S., Kastelein, R.A. & Cua, D.J. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27, 17–23 (2006).
Gantner, B.N., Simmons, R.M., Canavera, S.J., Akira, S. & Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003).
Dillon, S. et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116, 916–928 (2006).
Du, Z. et al. Selective regulation of IL-10 signaling and function by zymosan. J. Immunol. 176, 4785–4792 (2006).
Riley, L.W. Of mice, men, and elephants: Mycobacterium tuberculosis cell envelope lipids and pathogenesis. J. Clin. Invest. 116, 1475–1478 (2006).
Acknowledgements
We thank F. Powrie (Sir William Dunn School of Pathology, University of Oxford, Oxford, UK) for the transfer of CD4dnTGFβRII breeder mice; H. Jani for initial help in preparation of spinal cords; and H. Boyes for assessing clinical scores in treated mice.
Author information
Authors and Affiliations
Contributions
M.V. did the experiments; R.J.H. assisted with RT-PCR; R.A.F. provided the CD4dnTGFβ RII mice; and B.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Cytokine mRNA induction after DC stimulation. (PDF 87 kb)
Rights and permissions
About this article
Cite this article
Veldhoen, M., Hocking, R., Flavell, R. et al. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol 7, 1151–1156 (2006). https://doi.org/10.1038/ni1391
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1391
This article is cited by
-
Astrocyte interferon-gamma signaling dampens inflammation during chronic central nervous system autoimmunity via PD-L1
Journal of Neuroinflammation (2023)
-
IL-17A Facilitates Entry of Autoreactive T-Cells and Granulocytes into the CNS During EAE
NeuroMolecular Medicine (2023)
-
Local inhibition of TGF-β1 signaling improves Th17/Treg balance but not joint pathology during experimental arthritis
Scientific Reports (2022)
-
Tanshinone IIA Ameliorates CNS Autoimmunity by Promoting the Differentiation of Regulatory T Cells
Neurotherapeutics (2020)
-
PACAP/PAC1 Regulation of Inflammation via Catecholaminergic Neurons in a Model of Multiple Sclerosis
Journal of Molecular Neuroscience (2019)