Abstract
The molecular mechanisms by which signaling via transforming growth factor-β (TGF-β) and interleukin 4 (IL-4) control the differentiation of CD4+ IL-9-producing helper T cells (TH9 cells) remain incompletely understood. We found here that the DNA-binding inhibitor Id3 regulated TH9 differentiation, as deletion of Id3 increased IL-9 production from CD4+ T cells. Mechanistically, TGF-β1 and IL-4 downregulated Id3 expression, and this process required the kinase TAK1. A reduction in Id3 expression enhanced binding of the transcription factors E2A and GATA-3 to the Il9 promoter region, which promoted Il9 transcription. Notably, Id3-mediated control of TH9 differentiation regulated anti-tumor immunity in an experimental melanoma-bearing model in vivo and also in human CD4+ T cells in vitro. Thus, our study reveals a previously unrecognized TAK1–Id3–E2A–GATA-3 pathway that regulates TH9 differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Schmitt, E. et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-β and IL-4, and is inhibited by IFN-γ. J. Immunol. 153, 3989–3996 (1994).
Veldhoen, M. et al. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9, 1341–1346 (2008).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+Foxp3− effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Nicolaides, N.C. et al. Interleukin 9: a candidate gene for asthma. Proc. Natl. Acad. Sci. USA 94, 13175–13180 (1997).
Cheng, G. et al. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am. J. Respir. Crit. Care Med. 166, 409–416 (2002).
Staudt, V. et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33, 192–202 (2010).
Jäger, A., Dardalhon, V., Sobel, R.A., Bettelli, E. & Kuchroo, V.K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 183, 7169–7177 (2009).
Singh, T.P. et al. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS ONE 8, e51752 (2013).
Purwar, R. et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 18, 1248–1253 (2012).
Lu, Y. et al. Th9 cells promote antitumor immune responses in vivo. J. Clin. Invest. 122, 4160–4171 (2012).
Goswami, R. et al. STAT6-dependent regulation of Th9 development. J. Immunol. 188, 968–975 (2012).
Chang, H.C. et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 11, 527–534 (2010).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Maruyama, T. et al. Control of the differentiation of regulatory T cells and TH17 cells by the DNA-binding inhibitor Id3. Nat. Immunol. 12, 86–95 (2011).
Li, H., Dai, M. & Zhuang, Y.A. T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity 21, 551–560 (2004).
Jones-Mason, M.E. et al. E protein transcription factors are required for the development of CD4+ lineage T cells. Immunity 36, 348–361 (2012).
Liu, Y. et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Derynck, R. & Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Mu, Y., Gudey, S.K. & Landstrom, M. Non-Smad signaling pathways. Cell Tissue Res. 347, 11–20 (2012).
Choi, M.E., Ding, Y. & Kim, S.I. TGF-β signaling via TAK1 pathway: role in kidney fibrosis. Semin. Nephrol. 32, 244–252 (2012).
Tanaka, K. et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-γ on STAT3 and Smads. J. Immunol. 180, 3746–3756 (2008).
Tamiya, T. et al. Smad2/3 and IRF4 play a cooperative role in IL-9-producing T cell induction. J. Immunol. 191, 2360–2371 (2013).
Wang, A. et al. Cutting edge: Smad2 and Smad4 regulate TGF-β-mediated Il9 gene expression via EZH2 displacement. J. Immunol. 191, 4908–4912 (2013).
Yamashita, M. et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol. Cell 31, 918–924 (2008).
Xiao, X. et al. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat. Immunol. 13, 981–990 (2012).
Murre, C. Helix-loop-helix proteins and lymphocyte development. Nat. Immunol. 6, 1079–1086 (2005).
Murre, C. et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58, 537–544 (1989).
Wilhelm, C. et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12, 1071–1077 (2011).
Jash, A. et al. Nuclear factor of activated T cells 1 (NFAT1)-induced permissive chromatin modification facilitates nuclear factor-κB (NF-κB)-mediated interleukin-9 (IL-9) transactivation. J. Biol. Chem. 287, 15445–15457 (2012).
Tan, C. et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J. Immunol. 185, 6795–6801 (2010).
Yang, X. et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 18, 1280–1291 (1999).
Konkel, J.E. et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol. 12, 312–319 (2011).
Wei, G., Hu, G., Cui, K. & Zhao, K. Genome-wide mapping of nucleosome occupancy, histone modifications, and gene expression using next-generation sequencing technology. Methods Enzymol. 513, 297–313 (2012).
Acknowledgements
We thank J.E. Konkel and C. Chia for critical reading of the manuscript; Y. Zhuang (Duke University) for Id3f/fCd4-Cre+ mice; X.C. Li (Harvard Medical School) for antigen-presenting cells with transgenic expression of the ligand for OX40; Y. Wan (University of North Carolina) for Tak1f/fER-Cre mice; and A. Yoshimura (Keio University of Medicine) for Smad2f/fLck-Cre+ mice. Supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research (US National Institutes of Health) and the JSPS Research Fellowship Program for Japanese Biomedical and Behavioral Researchers at the US National Institutes of Health (H.N).
Author information
Authors and Affiliations
Contributions
H.N., D.Z. and T.M. designed and performed most of the experiments, interpreted the data and drafted the paper; H.C., M.I., L.D., P.Z., E.T., W.J., B.A. and N.G. performed experiments; K.C. performed experiments with ChIP-Seq; Q.C. and L.S. provided scientific input; K.Z. supervised and designed experiments with ChIP-Seq and interpreted data; and W.C. conceived of and supervised the research, designed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
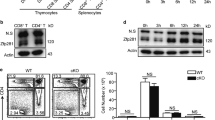
Supplementary Figure 1 Phenotype of Id3f/fCd4-Cre+ T cells.
Naive CD4+CD25- T cells were isolated from Id3f/f Cd4-Cre+ (n=2) or Id3+/+ Cd4-Cre+ (n=2) mice and cultured with anti-CD3 and anti-CD28 antibodies. (a) The surface expression of activation associated markers CD25 and CD69 were determined by flow cytometry at 0 hr (freshly isolated) and 16 hr after stimulation with anti-CD3 and-CD28 antibodies (TCR). (b) Il2 mRNA expression at 16 h after TCR stimulation. (c) The frequency of apoptotic and dead CD4+ T cells at 16 h after TCR stimulation was assessed with Annexin V and 7-AAD staining. (d) Naïve CD4+CD25- T cells were labeled with CFSE and cultured with (Id3f/f Cd4-Cre+ and Id3+/+ Cd4-Cre+) or without (Med) TCR stimulation for 3 days, T cell proliferation was determined by CFSE dilution.
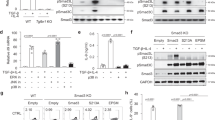
Supplementary Figure 2 Downstream signaling of TGF-β during TH9 differentiation.
(a) Intracellular staining of IFN-γ, IL-4, Foxp3, and IL-17 in CD4+ T cells isolated from tamoxifen- or oil-treated TgfbrIIf/f ER-Cre+ mice and cultured with anti-CD3 and anti-CD28 with or without TGF-β1 plus IL-4 or IL-6. Data representative of two individual experiments. (b-e) Naïve CD4+CD25- T cells from the indicated Smad deficient mice and their respective controls were stimulated with anti-CD3 and anti-CD28 antibodies with TGF-β1 in the absence (lower panels in each group, Treg differentiation cultures) and presence (upper panels in each group, TH9 differentiation cultures) of IL-4 for 3 days. As indicated, TAK1 inhibitor was included in some cultures. Intracellular IL-9 and Foxp3 expression in CD4+ T cells were determined by flow cytometry analysis. The numbers in the figure represents the percentages of IL-9+ or Foxp3+ T cells within the gated CD4+ T cells. Data shown in the figure are representative of two (b,d,e) or four (c) independent experiments. Smad2 KO: Smad2f/f Lck-Cre+; Smad3 KO: Smad3 null mutation (Smad3-/-); Smad4 KO: Smad4f/f Cd4-Cre+; Smad3/4 KO: Smad3-/-Smad4f/f Cd4-Cre+. Error bars represent mean±SD. **p<0.01, ***p<0.001 (Student’s t-test). (f) mRNA expression of Id3 in wild-type or Smad3/4 deficient CD4+ T cells cultured with anti-CD3 and anti-CD28 with TGF-β1 plus IL-4 for 24h. Error bars represent mean±SD. Data presented relative to Hprt expression and is representative of two independent experiments.
Supplementary Figure 3 Blockade of TAK1 fails to significantly affect the differentiation of Treg cells and TH17 cells.
(a) Naive CD4+ T cells were cultured under Treg (left) or TH17 (middle) polarizing conditions, or under neutral stimulation (right) in the absence or presence of TAK1 inhibitor as indicated. The frequency of Foxp3+ Treg cells, IL-17+ TH17 cells, and IFN-γ+ TH1 cells were determined by flow cytometry. (b) Naive CD4+ T cells from Tak1f/f ER-Cre+ mice were cultured with anti-CD3 and anti-CD28 and 4-hydroxytamoxifen (4-OHT) for overnight to delete TAK1 expression. TGF-β1 or TGF-β1 plus IL-6 were then added into the cells and cultured for additional 2.5 days. Upper panel: TAK1 protein expression was measured by flow cytometry after the culture with or without 4-OHT treatment. Lower panels: mRNA expression of Foxp3 and Il17a in the indicated conditions was determined by real-time RT-PCR analysis. Data presented relative to Hprt expression and represent at least three independent experiments. Error bars represent mean±SD. (c) Naive CD4+ T cells were cultured with OX40L-transgenic APCs plus anti-CD3, TGF-β1 plus IL-4 in the presence or absence of TAK1 inhibitor (left), and combined results of three individual experiments are indicated (right). Error bars represent mean±SD. **p<0.01, ***p<0.001 (Student’s t-test). (d) Time course change of Sfpi1 and Irf4 mRNA expression in wild-type naive CD4+ T cells cultured with anti-CD3 and anti-CD28 with or without TGF-β1 plus IL-4 or TAK1 inhibitor. Data presented relative to Hprt expression and represent two individual experiments combined. Error bars represent mean±SD.
Supplementary Figure 4 TH9 polarization does not affect the expression of E2A-encoding mRNA or E2A protein.
(a) mRNA expression of E2A (Tcfe2a) in wild-type naive CD4+ T cells cultured with anti-CD3 and anti-CD28 with or without TGF-β1 plus IL-4 for 24h. Data presented relative to Hprt expression and represent three individual experiments combined. Error bars represent mean±SD. (b) Western blotting of E47 expression in nuclear fraction and cytosol of naive CD4+ T cells cultured with anti-CD3 and anti-CD28 with or without TGF-β1 plus IL-4 for 24h. Images are representative of two independent experiments. (c) Naïve CD4+ T cells were isolated from wild-type mice and Il9 promoter reporter construct was transfected with control or E47-expressing vector. Luciferase assay was assessed after stimulation with TGF-β1 plus IL-4 for 24h. Data is representative of three individual experiments. Error bars represent mean±SD.
Supplementary Figure 5 GATA-3 is expressed in TH9 cells.
(a) mRNA expression of Gata3 and Il9 in wild-type naive CD4+ T cells cultured with anti-CD3 and anti-CD28 with or without TGF-β1 and/or IL-4 for 24h. (b) Intracellular staining of IL-9 and GATA-3 in wild-type naive CD4+ T cells cultured with anti-CD3 and anti-CD28 with or without TGF-β1 and/or IL-4 for 72h. Error bars represent mean±SD. **p<0.01, ***p<0.001 (Student’s t-test).
Supplementary Figure 6 Id3−/− mice spontaneously have IL-9+CD4+ cells and abundant circulating IL-9 protein.
(a) Intracellular staining of IL-9 in the spleen (SP), peripheral lymph nodes (LN), and lung of WT or Id3-/- mice in CD4+ cells. Left: Each plot represents individual mice. (SP, LN: n=8, Lung: n=5). Right: Representative of FACS plots. (b) IL-9 levels in the sera were measured by ELISA and data were shown as relative OD value, the mean OD of Id3+/+ mice in each measurement was set as 1. Error bars represent mean±SD. *p<0.05, ***p<0.001 (unpaired Student’s t-test).
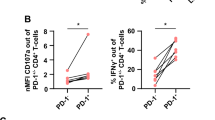
Supplementary Figure 7 IL-9 produced by Id3f/fCd4-Cre+ CD4+ T cells is involved in anti-tumor immunity.
(a) Tumor growth over time in Rag1-/- mice given intravenous transfer of TGFβ1-treated naïve CD4+CD25- T cells from Id3f/f Cd4-Cre+ or Id3+/+ Cd4-Cre+ mice along with simultaneous subcutaneous injection of B16 melanoma cells on day 0, then treated with anti-IL-9 (α-IL-9) or isotype-matched control (Ctrl Ab) antibodies every 3 d from day 0. (b) Tumor growth over time in Rag1-/- mice given intravenous transfer of naïve CD4+CD25- T cells, which were isolated from Tak1f/f ER-Cre+ mice and cultured with anti-CD3 and anti-CD28 antibodies in the absence (WT) or presence (TAK1 cKO) of 4-hydroxy tamoxifen (4-OHT) for overnight to delete TAK1, followed by the stimulation with TGF-β1 plus IL-4 for additional 3 d. The mice were subcutaneously injected with B16 melanoma cells on day 0, then treated with anti-IL-9 (α-IL-9) or isotype-matched control (Ctrl Ab) antibodies every 3 d from day 0. (c) Intracellular staining of Foxp3, IFN-γ, IL-17, and IL-4 in intratumoral CD4+ T cells in representative mice from each group as in a. (d) Intracellular staining of Foxp3, IFN-γ, IL-17, and IL-4 in intratumoral CD4+ T cells in representative mice from each group as in b. Error bars represent mean ± SEM of the tumor volumes of individual mice (n=4 per group in (a) and WT mice per condition in (b) or n=3 TAK1 cKO mice per condition in (b). *p<0.05, **p<0.01 (Id3f/f Cd4-Cre+ + ctrl Ab vs Id3+/+ Cd4-Cre+ + ctrl Ab in (a) or WT + ctrl Ab vs WT + α-IL-9 in (b)); ##p<0.01 (Id3f/f Cd4-Cre+ + ctrl Ab vs Id3f/f Cd4-Cre+ + α-IL-9). (unpaired Student’s t-test).
Supplementary Figure 8 Induction of Il9 by stimulation with anti-CD3 in human naive T cells.
(a) Il9 mRNA expression in naive CD4+CD25-CD45RA+ cells isolated from human peripheral blood mononuclear cells and cultured with anti-CD3 with various combination of TGF-β1, IL-4, TGF-β receptor inhibitor, and anti-IL-4 for 48h. (b) Id3 mRNA expression in naive CD4+ T cells treated with Id3-specific or control siRNA, assessed after TCR stimulation with or without TGF-β1 plus IL-4 for 48h. mRNA expression was presented relative to Gapdh expression. Data representative of at least three individual experiments. Error bars represent mean ± SD.
Supplementary Figure 9 Proposed model for the TAK1–Id3–E2A–GATA-3 pathway in the regulation of Il9 transcription in T cells.
TGF-β1 and IL-4 signaling downregulates Id3 expression through TAK1 activity in naïve CD4+ T cells. The reduction of Id3 expression enhances E2A and also GATA-3 binding to Il9 promoter to transactivate Il9 gene. In addition, IL-4 signaling upregulates GATA-3 expression which binds to Il9 promoter to promote/amplify Il9 gene activation. IL-4 may downregulate Id3 expression at earlier time upon TCR stimulation (< 3 h) through TAK1-indepednent pathway, which itself may be insufficient, but helpful in TH9 cell differentiation, but the exact mechanisms remain unknown. In addition to Id3-E2A mediated pathway, TAK1 may also promote Il9 gene activation by Id3-independent mechanisms. TGF-β1 signaling is absolutely required for Il9 gene activation, however, Smad-mediated classic pathway probably plays a less important role than TAK1-mediated pathway, as deficiency of Smad2, Smad3 and 4 in T cells resulted in a partial decrease in their differentiation into TH9 cells, while inhibition of TAK1 activity or deletion of TAK1 expression almost completely blocked TH9 differentiation. (Black solid lines indicate evidence available; black dotted lines indicate no evidence available in the present study; down arrow indicates down-regulation).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1498 kb)
Rights and permissions
About this article
Cite this article
Nakatsukasa, H., Zhang, D., Maruyama, T. et al. The DNA-binding inhibitor Id3 regulates IL-9 production in CD4+ T cells. Nat Immunol 16, 1077–1084 (2015). https://doi.org/10.1038/ni.3252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3252
This article is cited by
-
Dynamic chromatin accessibility licenses STAT5- and STAT6-dependent innate-like function of TH9 cells to promote allergic inflammation
Nature Immunology (2023)
-
Opposing functions of circadian protein DBP and atypical E2F family E2F8 in anti-tumor Th9 cell differentiation
Nature Communications (2022)
-
IL-9 and IL-9-producing cells in tumor immunity
Cell Communication and Signaling (2020)
-
Depletion of ID3 enhances mesenchymal stem cells therapy by targeting BMP4 in Sjögren’s syndrome
Cell Death & Disease (2020)
-
The Role of Interleukin-9 in Cancer
Pathology & Oncology Research (2020)