Abstract
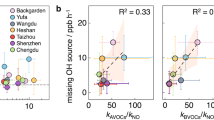
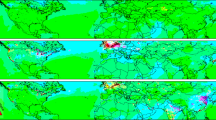
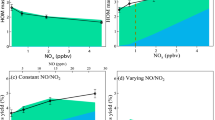
The removal of trace gases from the troposphere is, in most cases, initialized by reactions with hydroxyl radicals, and the products of these reactions are eventually deposited on the Earth's surface. The concentration of these hydroxyl radicals is therefore a measure of atmospheric self-cleansing. In theory, hydroxyl-radical concentrations can be enhanced by the recycling of some of the reaction products. The only known efficient recycling process involves nitrogen oxide and leads to production of ozone, yet observations in regions with high hydrocarbon and low nitrogen oxide concentrations show substantially elevated hydroxyl-radical concentrations, up to ten times higher than expected. If we normalize observed hydroxyl-radical concentrations to the maximum achievable in model calculations with variable nitrogen oxide concentrations, this photochemical coordinate system uncovers a common feature in almost all of these observations: even in the presence of inadequate amounts of nitrogen oxides, hydroxyl-radical concentrations are enhanced to the theoretical maximum obtainable at very much higher nitrogen oxide concentrations. This means that this important part of the self-cleansing capability of the atmosphere is working at maximum efficiency even in regions with a high burden of biogenic hydrocarbons and low nitrogen oxide concentration. Since these processes do not involve nitrogen oxides, tropospheric ozone production is greatly reduced compared with the expectation from current theory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levy, H. II Normal atmosphere: Large radical and formaldehyde concentrations. Science 173, 141–143 (1971).
Ehhalt, D. H. Photooxidation of trace gases in the troposphere. Phys. Chem. Chem. Phys. 1, 5401–5408 (1999).
Liu, S. C. et al. Ozone production in the rural troposphere and the implications for regional and global ozone distributions. J. Geophys. Res. 92, 4191–4207 (1987).
Ehhalt, D. H. & Rohrer, F. Dependence of the OH concentration on solar UV. J. Geophys. Res. 105, 3565–3571 (2000).
Brauers, T., Hausmann, M., Bister, A., Kraus, A. & Dorn, H. P. OH radicals in the boundary layer of the Atlantic Ocean: 1. Measurements by long-path laser absorption spectroscopy. J. Geophys. Res. 106, 7399–7414 (2001).
Whalley, L. K. et al. The chemistry of OH and HO2 radicals in the boundary layer over the tropical Atlantic Ocean. Atmos. Chem. Phys. 10, 1555–1576 (2010).
Mao, J. Q. et al. Atmospheric oxidation capacity in the summer of Houston 2006: Comparison with summer measurements in other metropolitan studies. Atmos. Environ. 44, 4107–4115 (2009).
Dusanter, S. et al. Measurements of OH and HO2 concentrations during the MCMA-2006 field campaign — Part 2: Model comparison and radical budget. Atmos. Chem. Phys. 9, 6655–6675 (2009).
Kanaya, Y. et al. Urban photochemistry in central Tokyo: 1. Observed and modeled OH and HO2 radical concentrations during the winter and summer of 2004. J. Geophys. Res. 112, D21312 (2007).
Ren, X. R. et al. OH and HO2 chemistry in the urban atmosphere of New York City. Atmos. Environ. 37, 3639–3651 (2003).
Tan, D. et al. HOx budgets in a deciduous forest: Results from the PROPHET summer 1998 campaign. J. Geophys. Res. 106, 24407–24427 (2001).
Lelieveld, J. et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 452, 737–740 (2008).
Hofzumahaus, A. et al. Amplified trace gas removal in the troposphere. Science 324, 1702–1704 (2009).
Whalley, L. K. et al. Quantifying the magnitude of a missing hydroxyl radical source in a tropical rainforest. Atmos. Chem. Phys. 11, 7223–7233 (2011).
Lu, K. D. et al. Missing OH source in a suburban environment near Beijing: observed and modelled OH and HO2 concentrations in summer 2006. Atmos. Chem. Phys. 13, 1057–1080 (2013).
Archibald, A. T. et al. Impacts of HOx regeneration and recycling in the oxidation of isoprene: Consequences for the composition of past, present and future atmospheres. Geophys. Res. Lett. 38, 6655–6675 (2011).
Peeters, J. & Müller, J-F. HOx radical regeneration in isoprene oxidation via peroxy radical isomerisations. II: experimental evidence and global impact. Phys. Chem. Chem. Phys. 12, 14227–14235 (2010).
Taraborrelli, D. et al. Hydroxyl radical buffered by isoprene oxidation. Nature Geosci. 5, 190–193 (2012).
Crounse, J. D., Paulot, F., Kjaergaard, H. G. & Wennberg, P. O. Peroxy radical isomerization in the oxidation of isoprene. Phys. Chem. Chem. Phys. 13, 13607–13613 (2011).
Fuchs, H. et al. Experimental evidence for efficient hydroxyl radical regeneration in isoprene oxidation. Nature Geosci. 6, 1023–1026 (2013).
Lu, K. D. et al. Observation and modelling of OH and HO2 concentrations in the Pearl River Delta 2006: a missing OH source in a VOC rich atmosphere. Atmos. Chem. Phys. 12, 1541–1569 (2012).
Rohrer, F. & Berresheim, H. Strong correlation between levels of tropospheric hydroxyl radicals and solar ultraviolet radiation. Nature 442, 184–187 (2006).
Eisele, F. L. et al. Understanding the production and interconversion of the hydroxyl radical during the Tropospheric OH Photochemistry Experiment. J. Geophys. Res. 102, 6457–6465 (1997).
Mao, J. et al. Insights into hydroxyl measurements and atmospheric oxidation in a California forest. Atmos. Chem. Phys. 12, 8009–8020 (2012).
Holland, F., Hofzumahaus, A., Schäfer, J., Kraus, A. & Pätz, H. W. Measurements of OH and HO2 radical concentrations and photolysis frequencies during BERLIOZ. J. Geophys. Res. 108, 8246 (2003).
Peeters, J., Nguyenz, T. L. & Vereecken, L. HOx radical regeneration in the oxidation of isoprene. Phys. Chem. Chem. Phys. 11, 5935–5939 (2009).
Kubistin, D. et al. Hydroxyl radicals in the tropical troposphere over the Suriname rainforest: comparison of measurements with the box model MECCA. Atmos. Chem. Phys. 10, 9705–9728 (2010).
Tan, D. et al. HOx budgets in a deciduous forest: Results from the PROPHET summer 1998 campaign. J. Geophys. Res. 106, 24407–24427 (2001).
Acknowledgements
Discussions with D. H. Ehhalt, D. Klemp and D. Mihelcic are gratefully acknowledged. This work was supported by the National Natural Science Foundation of China (21190052 and 41121004) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB05010500). The research was also supported by the Collaborative Innovation Center for Regional Environmental Quality. Our colleague Theo Brauers sadly passed away on 21 February 2014. We would like to take this opportunity to express our sincere appreciation for his excellent work.
Author information
Authors and Affiliations
Contributions
F.R. and K.L. contributed equally to this work. All other authors also contributed extensively to the work presented in this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4170 kb)
Rights and permissions
About this article
Cite this article
Rohrer, F., Lu, K., Hofzumahaus, A. et al. Maximum efficiency in the hydroxyl-radical-based self-cleansing of the troposphere. Nature Geosci 7, 559–563 (2014). https://doi.org/10.1038/ngeo2199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2199
This article is cited by
-
Satellite isoprene retrievals constrain emissions and atmospheric oxidation
Nature (2020)
-
Urban pollution greatly enhances formation of natural aerosols over the Amazon rainforest
Nature Communications (2019)