There’s a new way to eat carbon dioxide. Researchers have built an artificial version of a chloroplast, the photosynthetic structures inside plant cells. It uses sunlight and a laboratory-designed chemical pathway to turn CO2 into sugar.
Artificial photosynthesis could be used to drive tiny, non-living, solar-powered factories that churn out therapeutic drugs. And because the new chemical pathway is more efficient than anything nature has evolved, the team hopes that a similar process could some day even help to remove CO2 from the atmosphere — although it is not clear whether it could be turned into a large-scale, economically feasible operation. The work was published in Science on 7 May1.
Nature has evolved six pathways for ‘fixing’ CO2 — that is, turning it into sugar using enzymes that harness solar or chemical energy. In 2016, Tobias Erb, a synthetic biologist at the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, and his colleagues designed a seventh2. “We simply used thermodynamic and kinetic considerations to ask if we could rethink CO2 fixation and make it more efficient,” says Erb. They named the pathway the CETCH cycle — a complicated network of enzymes that is 20% more energy efficient than the pathway used in natural forms of photosynthesis.
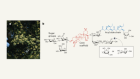
But it wasn’t clear whether the CETCH cycle would be compatible with the rest of a living cell’s machinery. To explore that possibility, Erb’s colleague Tarryn Miller turned to spinach. She extracted light-harvesting membranes from chloroplasts, the photosynthetic organelles common to all plants, and placed them in a reaction vessel alongside the 16 enzymes of their CETCH cycle. After some tweaking, Erb, Miller and their collaborators found that they could get the spinach membranes and their CETCH cycle enzymes to function together.
They had effectively created an artificial chloroplast, in which spinach chloroplast membranes harvest solar energy before the synthetic CETCH cycle enzymes use that energy to break down CO2. The enzymes convert the CO2 into a molecule called glycolate that can be used as a feedstock for making useful organic products.
“It’s a profound discovery,” says Paul King, a physical biochemist at the National Renewable Energy Laboratory in Golden, Colorado, who wasn’t involved in the study.
Although it’s just a proof of principle, it’s already possible to think of ways in which the artificial chloroplasts could be put to work, the authors say. Because of advances in synthetic biology, microbes can now be engineered to churn out useful molecules such as pharmaceutical drugs. But there are limits to what can be synthesized inside living cells. Erb says that the artificial chloroplasts could power non-living mini-reactors to produce molecules that living cells cannot.
They might be able to do so more efficiently than microbes can, says Kate Adamala, a synthetic biologist at the University of Minnesota in Minneapolis. “Natural cells spend a lot of energy on staying alive, while synthetic [systems] do not need to grow, reproduce or maintain any life-like functions,” she says. This means that the entire ‘metabolism’ of a synthetic system could be focused on producing valuable chemicals. Adamala says it is even possible to imagine artificial chloroplasts having a role in sequestering atmospheric CO2.
But there are problems to address before these applications can become reality. For example, the spinach membranes within the artificial chloroplasts function for just a few hours before they begin to degrade, limiting the working life of the system. And growing spinach and extracting membranes from its cells is relatively time consuming. “Using chloroplast extracts is not the smartest thing to upscale,” says Erb. Because of this, his team is also developing artificial systems to replace the spinach membranes.
There is also the tantalizing possibility of using the artificial chloroplasts to build fully synthetic organisms — cells assembled in the lab from the basic biological building blocks of life — although, again, there are challenges to address.
“We might be able to use the chloroplast mimics as an energy production system for artificial cells,” says Yutetsu Kuruma, a synthetic biologist at the Tokyo Institute of Technology. But in order to do so, he says that it would be helpful for the artificial chloroplasts to have some ability to self-repair and self-reproduce, like natural chloroplasts can. This is something they can’t do yet.
But this hasn’t deterred Erb and his colleagues from beginning experiments with synthetic cells. The team has begun collaborating with researchers at the J. Craig Venter Institute in La Jolla, California, who in 2016 built tiny synthetic cells containing the minimal number of genes for life. The plan is to put the CETCH cycle inside the ‘minimal’ cells— which might be one small step towards making synthetic life that can feed itself by munching on CO2.
“Nature can be very conservative, it never explored the full range of photosynthesis options,” says Erb. “That’s what excites us: we can realize solutions nature has never touched on.”




![High carrier mobility along the [111] orientation in Cu2O photoelectrodes](https://media.nature.com/w140h79/springer-static/image/art%3A10.1038%2Fs41586-024-07273-8/MediaObjects/41586_2024_7273_Fig1_HTML.png)


